|
Abstract
Objectives: To evaluate the immunohistochemical expression of estrogen receptors (ER) and progesterone receptors (PR) in colorectal adenoma and adenocarcinoma and to correlate this immunohistochemical expression with different clinicopathological parameters.
Methods: The study was retrospectively designed. A total of 86 tissue samples, including 33 paraffin blocks from patients with colorectal adenomas, 33 paraffin blocks from patients with colorectal adenocarcinomas and a control group of 20 samples of non-tumorous colonic tissue, were included in the study.
Results: The frequency of expression of ER and PR showed a gradual increase from control through adenoma to carcinoma. The frequencies of expression of ER in the control, adenoma and carcinoma were (10%, 15.15% and 42.42% respectively, p<0.001), while the frequency of expression for PR were (10%, 24.24% and 36.36% respectively, p<0.001). Strong ER and PR staining was mainly seen in carcinoma cases (42.42%, 36.36%, respectively) in comparison with adenoma (9.09%, 15.15%, respectively) and control (0%, 0%, respectively). The three digital parameters of ER and PR immunohistochemical expression (Area [A], Number of objects [N], and intensity [I]) were significantly increased in a sequence of normal mucosa-adenoma-carcinoma. There was a significant positive correlation between ER and PR in adenoma (r=0.312, p=0.034) and carcinoma (r=0.321, p=0.0398).
Conclusion: ER and PR expression increased in a sequence of; normal colonic mucosa-adenoma-carcinoma, and a positive correlation was observed between ER and PR expression in colonic adenoma and carcinoma specimen indicating that ER and PR may play a role in colorectal carcinogenesis.
Keywords: Estrogen receptors; Progesterone receptors; Colorectal adenoma; Colorectal carcinoma.
Introduction
The estrogen receptors (ER) and progesterone receptors (PR) belong to a superfamily of nuclear hormone receptors. These receptor proteins function as transcription factors when they are bound to their respective ligands. Since the original cloning of complementary DNAs (cDNAs) for ER8,9 and PR,10; an explosion of information has transpired in the field of steroid hormone action. These receptors share a common structural and functional organization.1
Colorectal cancer incidence has been reported to be 35% lower in women than in men.2 Although the basis for this difference is unknown, gonadal steroids are considered a contributing factor. The correlation in mortality data between breast cancer and colonic cancer, and the increased incidence of colonic cancer in individual women with breast cancer suggest common factors in their etiology. The protective effect of increasing parity from colonic cancer, similar to breast cancer and relatively better prognosis in women may also imply a common role for sex steroid hormones. The potential importance of ER and PR is emphasized through evidence of protection by hormone replacement therapy (HRT) in women and, by suggesting that the anti-estrogen tamoxifen may enhance the risk of colorectal cancer. In the Women’s Health Initiative study; HRT (estrogen and progesterone) conferred a 40% reduction in colorectal cancer risk in post menopausal women. HRT also conferred protection against the incidence and size of adenomas, as well as the colorectal cancer precursor lesions. All together, these data suggest that estrogens and/or progestins protect against colorectal cancer.2-5 ER and PR levels in colonic cancers are usually lower than in mammary cancers and it may be difficult to detect immunohistochemical expression in gastrointestinal cancers with lower levels.4
The Nurses’ Health study (NHS) and Polyp Prevention Trial (PPT) prospectively evaluated the effect HRT on colorectal adenomatous polyps in postmenopausal women who had ever used HRT compared to non-users. The NHS reported a 26% risk reduction for developing large adenomas in current users compared with non-users.6 Estrogen and progesterone receptors are higher in concentrations in colon cancers than in adenomas.7
The aim of the present study is to evaluate the immunohistochemical expression of ER and PR in colorectal adenoma and adenocarcinoma and to correlate the immunohistochemical expression with different clinico-pathological parameters of colorectal adenomas and carcinomas.
Methods
The study was retrospectively designed. A total of 86 tissue samples (paraffin block and tissue biopsies) were included in the study. An informed consent was taken from patients and relatives of control (autopsy) cases.
Of the total number of tissue samples, 66 paraffin blocks from patients with colorectal tumors were collected from Gastroenterology and Hepatology Center, Gastroenterology unit at Al-Khadhmiya Teaching Hospital, Baghdad, Iraq, and private laboratories for the period 2006-2010, including 33 blocks from patients with colorectal adenoma and 33 blocks from patients with colorectal adenocarcinoma. The control group included 20 samples of non-tumorous colonic tissue taken from autopsy cases from the Institute of Forensic Medicine. These specimens were processed and paraffin embedded at the same center. The clinicopathological parameters were obtained from patients’ admission case sheets and pathology reports. Absolute confidentiality of the patients’ vital information was maintained for ethical purposes and ethical approval was obtained from institutions in which the study was conducted.
From each block; three sections of 5 µm thickness were taken, one section was stained with Hematoxylin and Eosin (H&E) and the other two sections were stained immunohistochemically using three steps; indirect streptavidin method for Monoclonal Mouse Anti-Human Estrogen Receptor (ER), clone 1D5, manufactured by DAKO, Denmark and Monoclonal Mouse Anti-Human Progesterone Receptor (PR), clone PgR 636, manufactured by DAKO, Denmark. Brown cytoplasmic and nuclear ER and PR staining was considered a positive reaction in colorectal tissue, (Figs. 1,2).8 Positive control for ER and PR was considered from breast carcinoma tissue. Technical negative control was obtained by omission of primary antibody.
For colorectal adenocarcinoma; H&E slides were revised for the type, grade and stage (according to Astler-Coller staging system). Additional histopathological features were studied including; intratumoral lymphocytic infiltration and lymphovascular permeation. For colorectal adenoma, H&E slides were revised for the type and grade of dysplasia.
Scoring of immunohistochemical staining was performed using specialized automated cellular image analysis system, Digimizer software, version 3.7.0.9 Each immunohistochemically stained slide was scanned by a light microscope (Proway, China) for the positive brown immunostaining and the three fields that best reflect the overall immunostaining were chosen and captured using a Sony digital camera (digital still camera DSH-H55).
To determine a cut-off value for intensity of immunostaining; photomicrographs presented at the website of NordiCQ showing different grades of brown color intensity (strong (+3) for dark brown; moderate (+2) for brown; weak (+1) for light brown to yellow) were analyzed by the digimizer software, and the digital value of intensity was classed into three categories: weak, moderate and strong, taking into consideration that the digital value of intensity of staining is inversely proportional to the digital number in the digimizer color scale.9 (Table 1)
Table 1: Determination of staining intensity cut-off value.9
|
Grade of intensity
|
Digital value of digimizer
|
|
Strong +3
|
0.00-0.291
|
|
Moderate +2
|
0.292-0.719
|
|
Weak +1
|
0.720-0.999
|
|
Negative
|
1.000
|
Data were analyzed using SPSS program (Statistical Package for Social Sciences) version 16 and Microsoft Office Excel 2007. Numeric data were expressed as mean±SEM; frequency was used to express discrete data. ANOVA test was used to analyze numeric data while Chi-square test was used to analyze discrete data, while the Benferroni test was used for multiple comparisons. Scattered graph was used to show the relation between various markers. A p-value less than 0.05 was considered significant. For the Digimizer software, the integrated statistics window displayed statistics (n, mean of area, mean of average intensity, Standard deviation (SD), minimum and maximum) of the measurements in the Measurements list; these measurements were saved as a Excel 2007 spreadsheet file.
Results
The results pertaining to clinicopathological parameters assessed in the studied patients are shown in Table 2.
In comparing the immunohistochemical expressions of ER and PR in colorectal carcinoma, adenoma and control group; the results revealed that the frequency of expressions of ER and PR showed a gradual increase from control through adenoma to carcinoma. The frequency for ER expression was 10%, 15.15% and 42.42% respectively, p<0.001, while the frequency for PR expression was 10%, 24.24% and 36.36% respectively, p<0.001, (Table 3). The classification of the ER and PR positive cases of carcinoma, adenoma and control groups into different grades of intensity (negative, weak, moderate, and strong) according to the tabulated values of NordiCQ laboratories showed that strong ER staining was mainly seen in carcinoma cases (n=14; 42.42%) compared with adenoma cases (n=3; 9.09%) and the control (n=0; 0%), (Fig. 1). Strong PR staining was mainly observed in carcinoma cases (n=12; 36.36%) in comparison with adenoma (n=5; 15.15%) and control (n=0; 0%). (Fig. 2, Table 4)
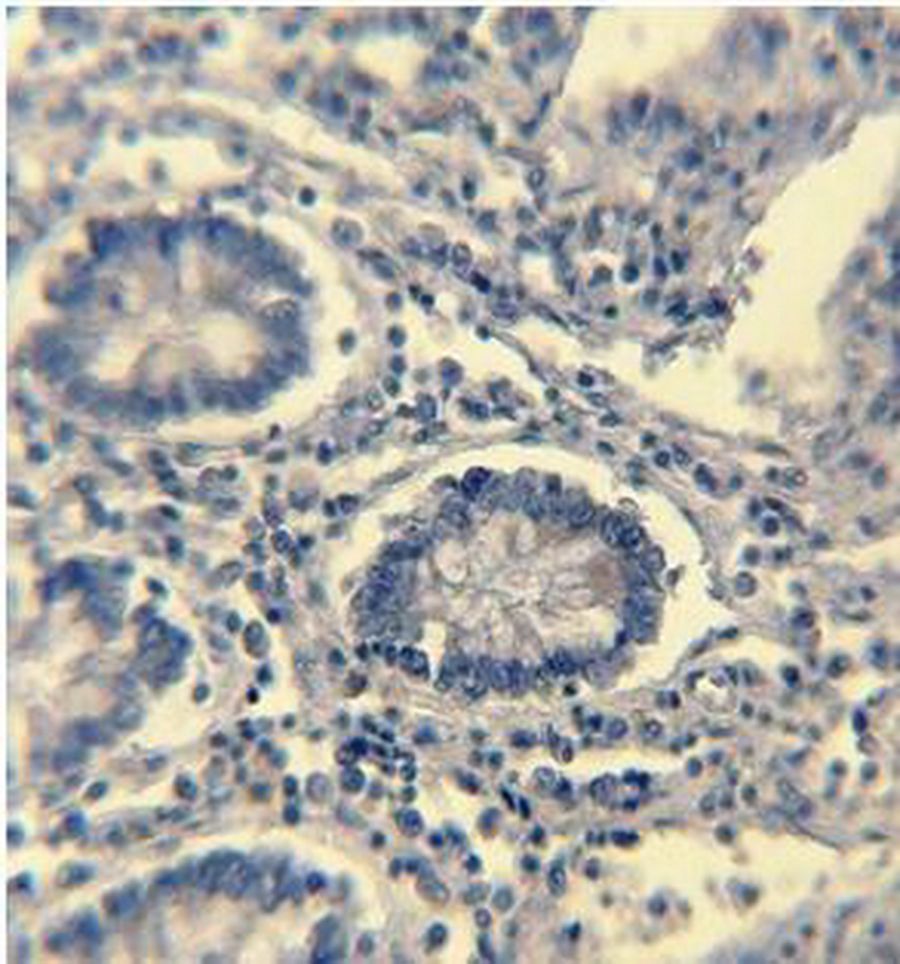
1A
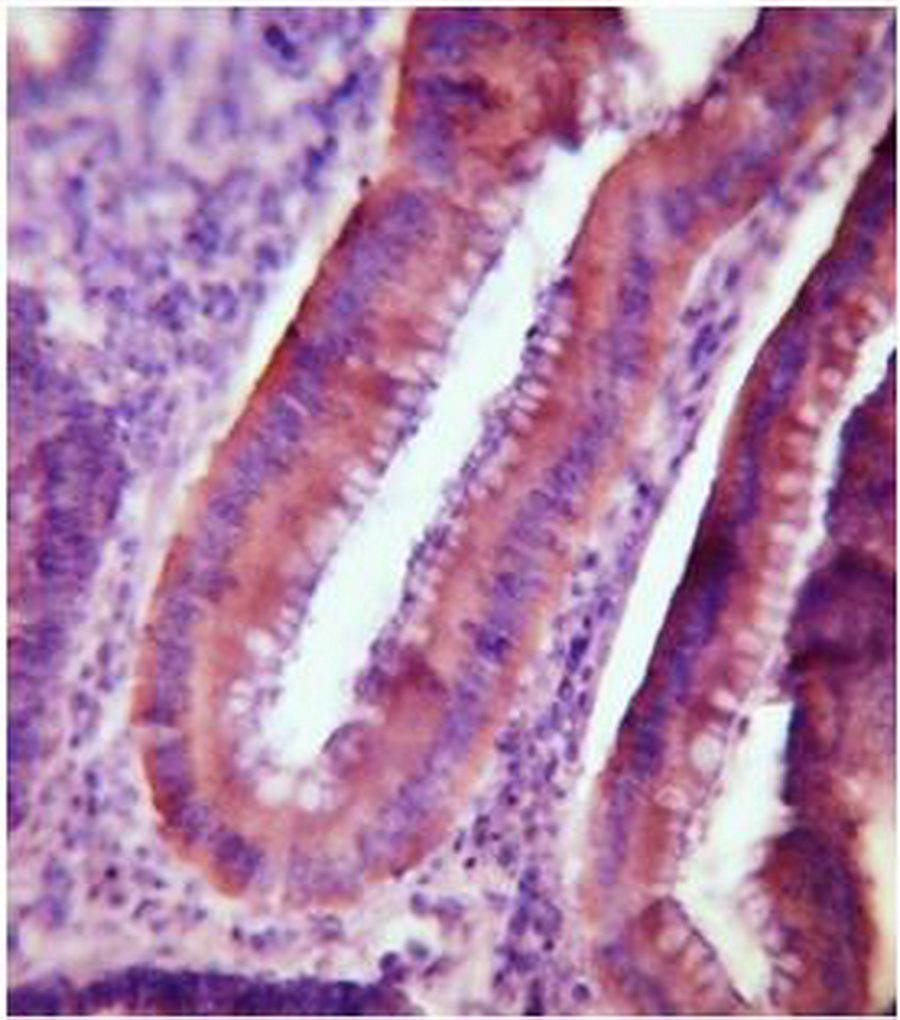
1B
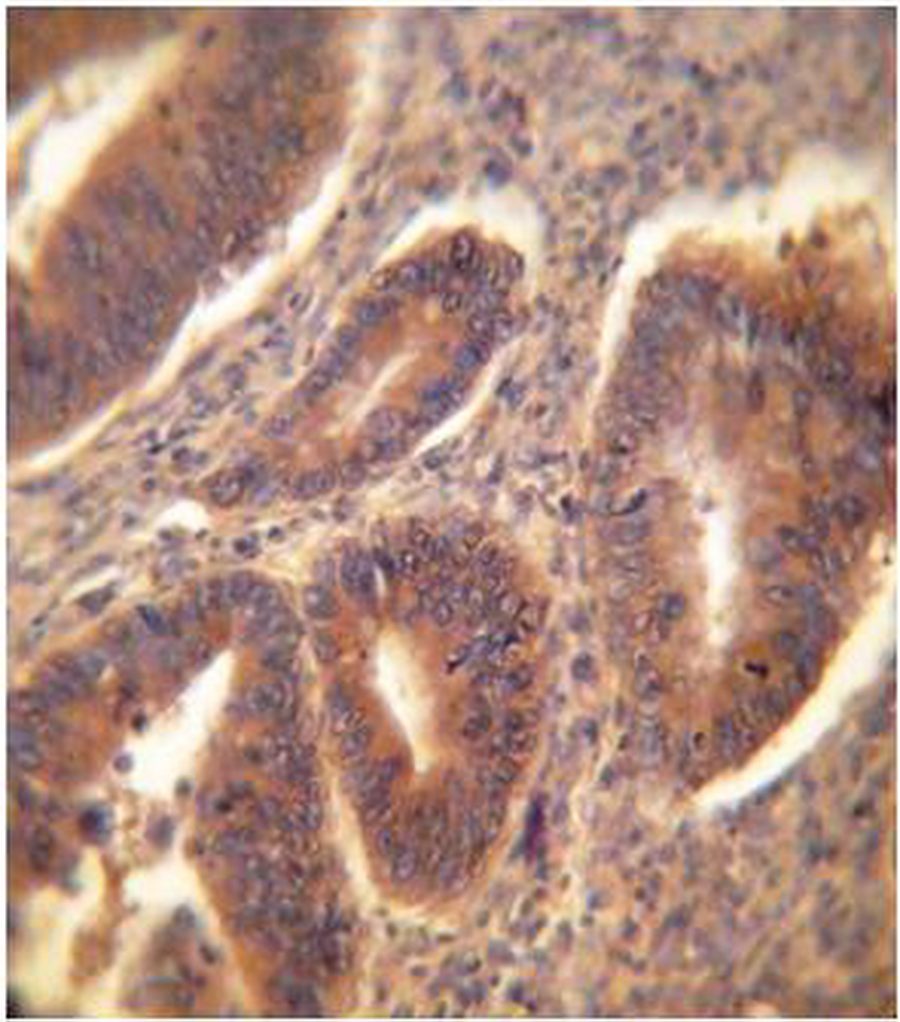
1C
Figure 1: ER Immunohistochemical expression. (1A) Normal colonic tissue showing ER-weak positive brown cytoplasmic staining at the base of the gland (40×). (1B) Tubular colonic adenoma with mild dysplasia showing ER-positive brown cytoplasmic staining with moderate intensity (40×). (1C) Well-differentiated colonic adenocarcinoma showing ER-positive brown cytoplasmic staining with strong intensity (40×).
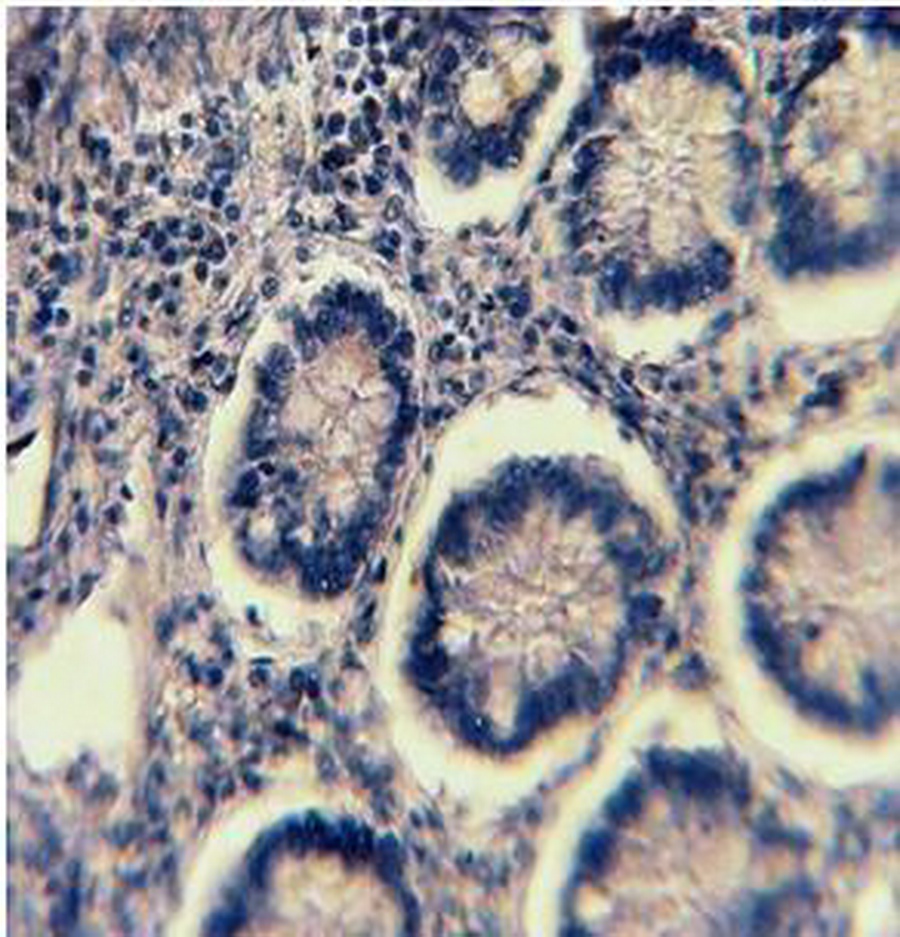
2A
2B
2C
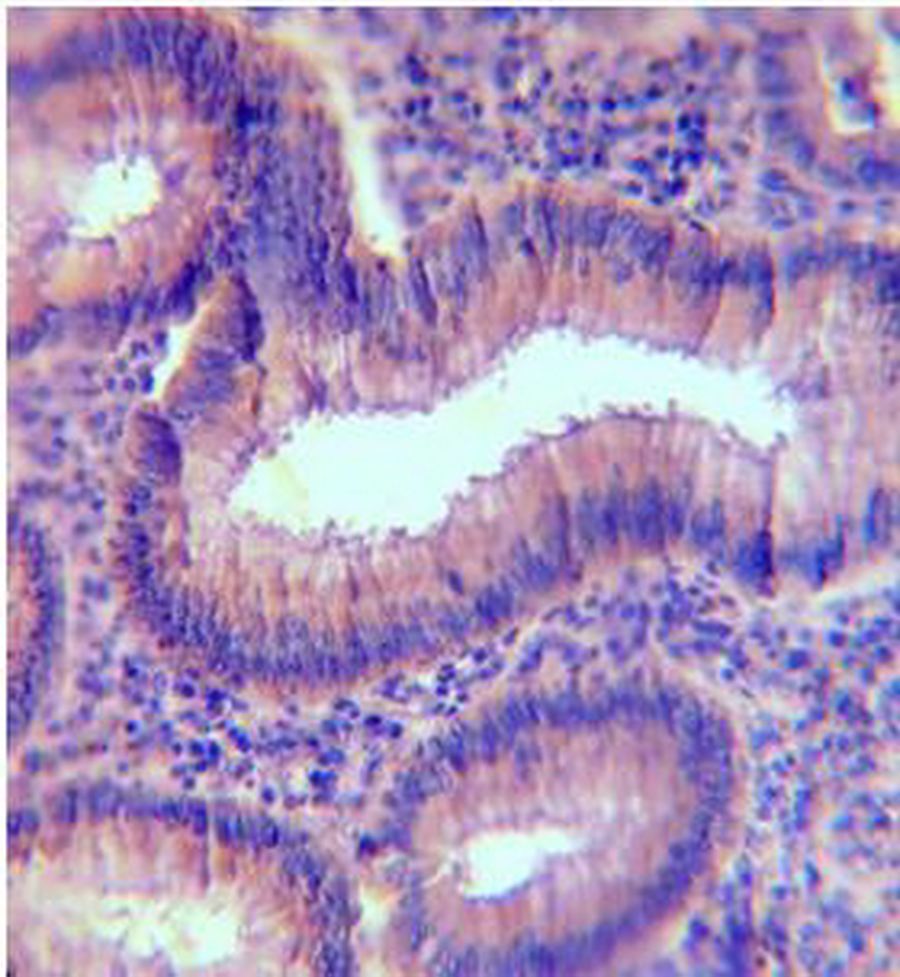
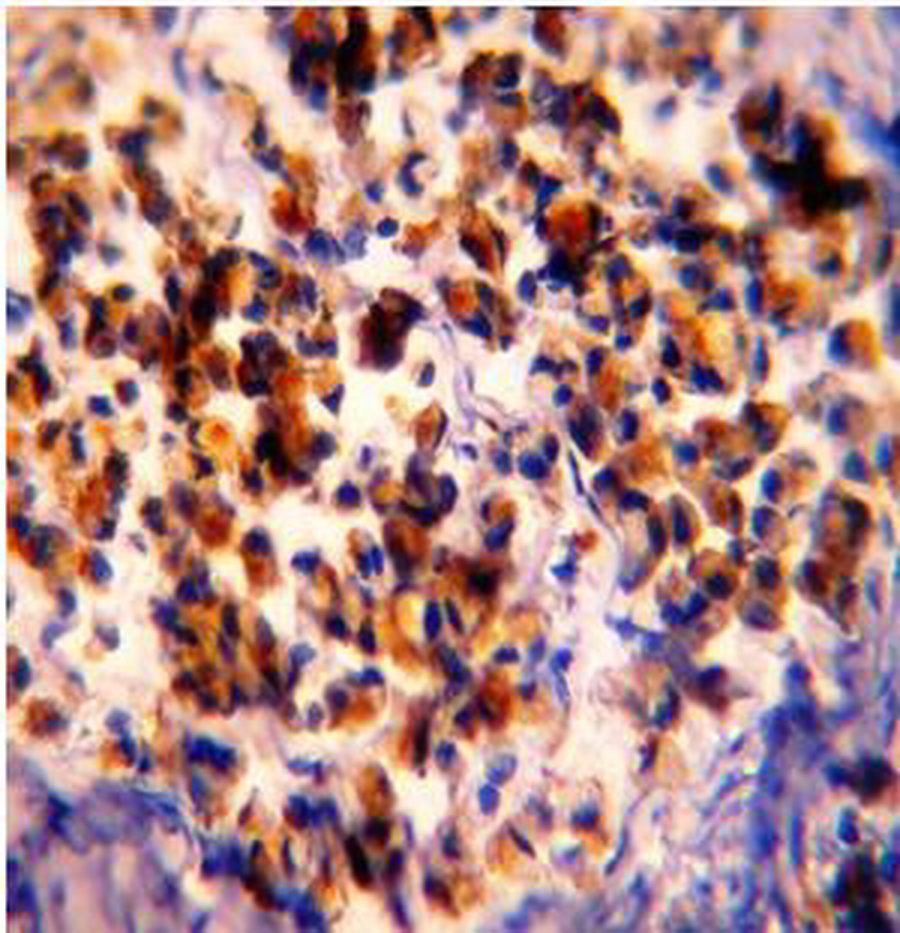
Figure 2: PR Immunohistochemical expression. (2A) Normal colonic showing PR-weak positive brown cytoplasmic staining at the base of the gland (40×). (2B) Tubular colonic adenoma with mild dysplasia showing PR-positive brown cytoplasmic staining with moderate intensity (40×). (2C) Signet ring colonic adenocarcinoma showing PR-positive brown cytoplasmic staining with strong intensity (40×).
The three digital parameters of ER and PR immunohistochemical expression [Area (A), Number of objects (N), and intensity (I)] were significantly increased in a sequence of; normal mucosa-adenoma-carcinoma. (Table 5)
Table 2: Clinicopathological parameters of patients studied.
|
Parameters
|
Adenoma (33 cases)
|
Carcinoma (33 cases)
|
|
Age (Years)
|
Mean±SD
|
51.12±2.75
|
56.03±2.37
|
|
|
Range
|
22 -80
|
27 - 80
|
|
Gender
|
Male
|
23 (69.69%)
|
19 (57.57%)
|
|
|
Female
|
10 (30.30%)
|
14 (42.42%)
|
|
|
M: F
|
2.3:1
|
1.35:1
|
|
Site
|
Right colon
|
14 (42.42%)
|
10 (30.30%)
|
|
|
Left colon
|
19 (57.57%)
|
23 (69.69%)
|
|
Size (cm)
|
Mean±SD
|
0.70±0.10
|
6.46±0.63
|
|
Histopathological types of adenoma
|
Tubular
|
11 (33.33%)
|
-
|
|
|
Tubulovillous
|
17 (51.51%)
|
-
|
|
|
Villous
|
5 (15.15%)
|
-
|
|
Dysplasia in adenomas
|
Mild
|
11 (33.33%)
|
-
|
|
|
Moderate
|
9 (27.27%)
|
-
|
|
|
Severe
|
13 (39.39%)
|
-
|
|
Number of adenomas
|
Solitary
|
25 (75.75%)
|
-
|
|
|
Multiple
|
8 (24.24%)
|
-
|
|
Gross morphology of carcinomas
|
Fungating
|
-
|
20 (60.60%)
|
|
|
Ulcerative
|
-
|
8 (24.24%)
|
|
|
Annular
|
-
|
5 (15.15%)
|
|
Histopathological types of carcinomas
|
Non-mucinous
|
-
|
25 (75.75%)
|
|
|
Mucinous
|
-
|
8 (24.24%)
|
|
Grade of carcinoma
|
Well-differentiated
|
-
|
2 (6.06%)
|
|
|
Moderately-differentiated
|
-
|
29 (87.87%)
|
|
|
Poorly -differentiated
|
-
|
2 (6.06%)
|
|
Stage of carcinoma(Astler-Coller)
|
B2
|
-
|
20 (60.6%).
|
|
|
C1
|
-
|
3 (9.09%)
|
|
|
C2
|
-
|
9 (27.27%)
|
|
|
D
|
-
|
1 (3.03%)
|
|
Lymph node involvement in carcinoma
|
Positive
|
-
|
13 (39.39%)
|
|
|
Negative
|
-
|
20 (60.60%)
|
|
Lymphovascular permeation in carcinoma
|
Positive
|
-
|
29 (87.87%)
|
|
|
Negative
|
-
|
4 (12.12%)
|
|
Lymphocytic infiltration in carcinoma
|
Positive
|
-
|
24 (72.72)
|
|
|
Negative
|
-
|
9 (27.27%)
|
Table 3: Frequency distribution of immunohistochemical expression of ER and PR in patients and control groups.
|
Marker
|
Expression
|
Adenoma No. (%)
|
Carcinoma No. (%)
|
Control No. (%)
|
p value
|
|
ER
|
Positive
|
5 (15.15%)
|
14 (42.42%)
|
2 (10%)
|
<0.001
|
|
|
Negative
|
28 (84.84%)
|
19 (57.57%)
|
18 (90%)
|
|
PR
|
Positive
|
8 (24.24%)
|
12 (36.36%)
|
2 (10%)
|
<0.001
|
|
|
Negative
|
25 (75.75%)
|
21 (63.63%)
|
18 (90%)
|
Table 4: Comparison of digimizer parameters (A, N, I) of ER and PR expression among patients and control groups.
|
Marker
|
Intensity score
|
Carcinoma
|
Adenoma
|
Control
|
p value
|
|
ER
|
Negative
|
19 (57.57%)
|
28 (84.84%)
|
18 (90%)
|
<0.001
|
|
|
Weak
|
0 (0%)
|
0 (0%)
|
2 (10%)
|
|
|
Moderate
|
0 (0%)
|
2 (6.06%)
|
0 (0%)
|
|
|
Strong
|
14 (42.42)
|
3 (9.09%)
|
0 (0%)
|
|
PR
|
Negative
|
21 (63.63%)
|
25 (75.75%)
|
18 (90%)
|
<0.001
|
|
|
Weak
|
0 (0%)
|
0 (0%)
|
2 (10%)
|
|
|
Moderate
|
0 (0%)
|
3 (9.09%)
|
0 (0%)
|
|
|
Strong
|
12 (36.36%)
|
5 (15.15%)
|
0 (0%)
|
(A): Area, (N): No. of objects, (I): intensity
Table 5: Comparison of digimizer parameters [Area (A), No. of objects (N), and intensity (I)] of ER and PR among patients and control groups.
|
Marker
|
Comparison
|
p value
|
|
A
|
N
|
I
|
|
ER
|
Carcinoma versus adenoma
|
<0.001
|
<0.001
|
0.009
|
|
Carcinoma versus control
|
0.024
|
0.010
|
<0.001
|
|
Adenoma versus control
|
<0.001
|
<0.001
|
<0.001
|
|
PR
|
Carcinoma versus adenoma
|
0.029
|
0.035
|
0.024
|
|
Carcinoma versus control
|
0.027
|
0.011
|
0.005
|
|
Adenoma versus control
|
<0.001
|
<0.001
|
<0.001
|
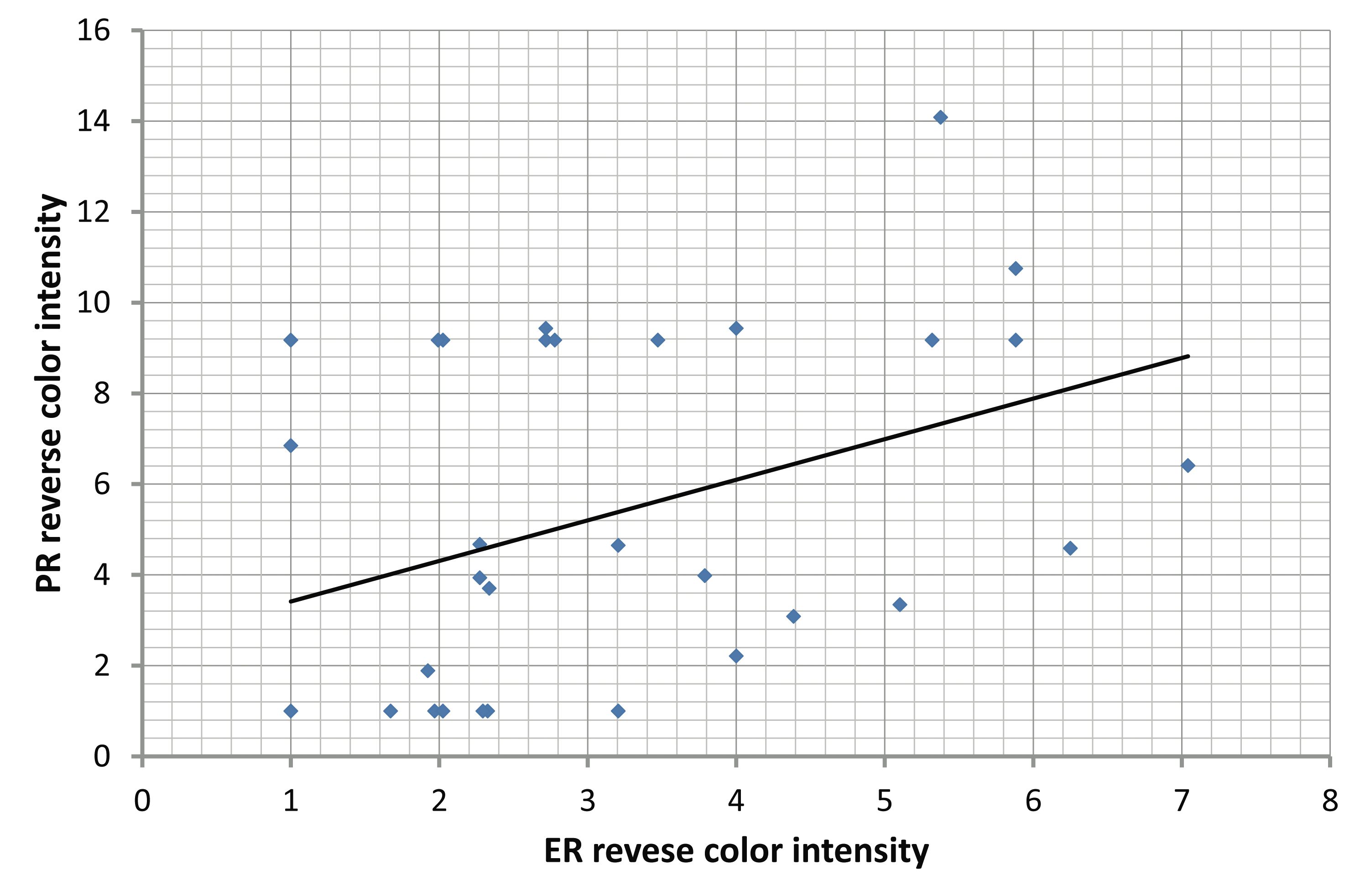
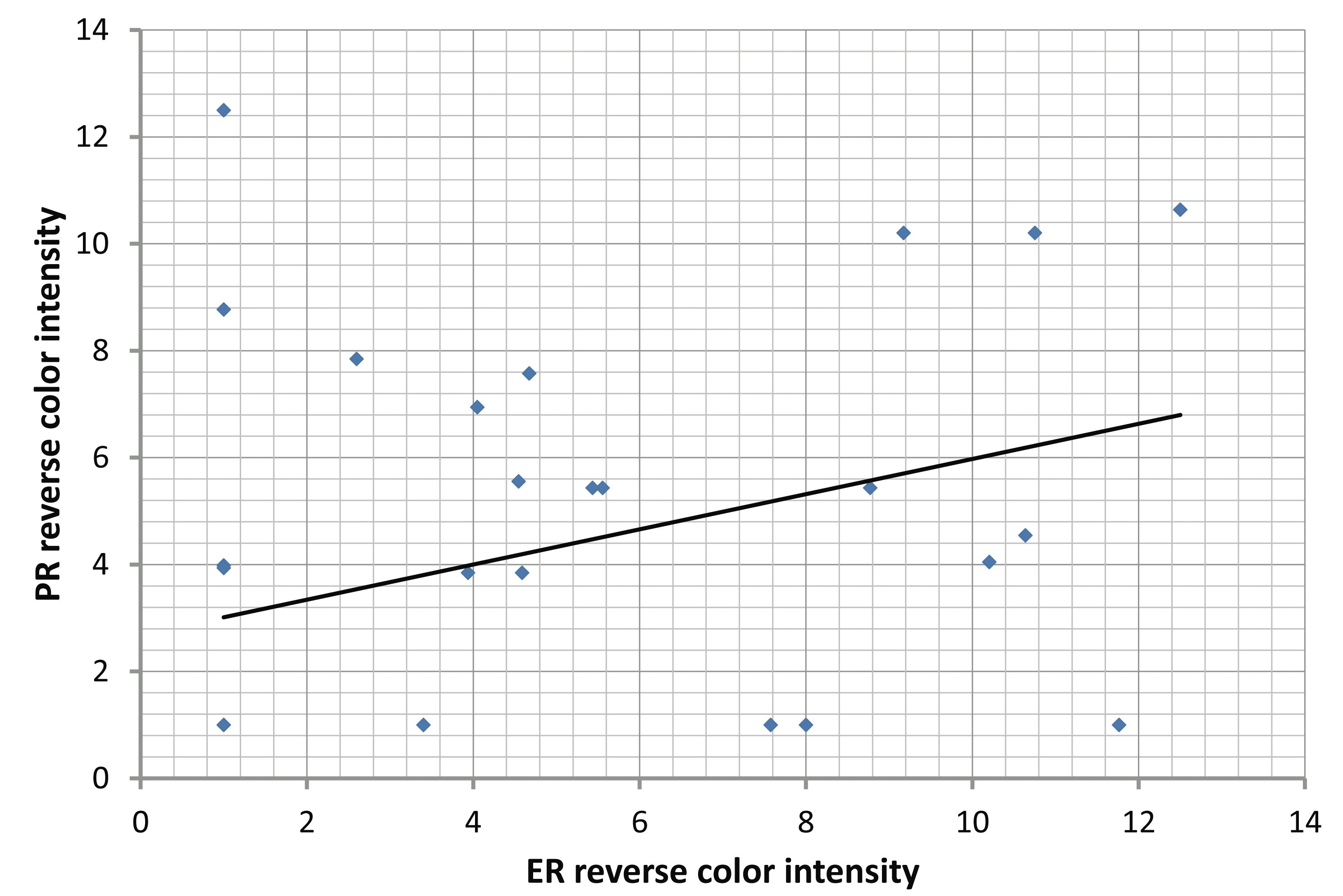
With regard to the correlation of ER and PR immunohistochemical expression with different clinicopathological parameters in colorectal adenomas and carcinomas; there was no significant correlation between the mean of the three digital parameters of the digimizer software (area [A], number of objects [N], and intensity [I]) of ER and PR with the clinicopathological parameters of colorectal adenomas and carcinomas, (Tables 6,7). However, there was a significant positive correlation between ER and PR in adenoma (r=0.312, p=0.034) and carcinoma (r=0.321, p=0.0398). (Fig. 3)
Table 6: Correlations of ER and PR immunohistochemical expression with different clinicopathological parameters in patients with colorectal adenomas.
|
Parameter
|
Area (A) (p value)
|
No. of objects (N) (p value)
|
Intensity (I) (p value)
|
|
ER
|
PR
|
ER
|
PR
|
ER
|
PR
|
|
Age
|
0.119
|
0.915
|
0. 122
|
0.309
|
0.756
|
0.432
|
|
Gender
|
0.532
|
0.992
|
0.942
|
0.284
|
0.964
|
0.874
|
|
Site
|
0.430
|
0.268
|
0.525
|
0.090
|
0.644
|
0.538
|
|
Size
|
0.522
|
0.109
|
0.319
|
0.743
|
0.624
|
0.399
|
|
No. of adenomas
|
0.064
|
0.162
|
0.627
|
0.867
|
0.057
|
0.210
|
|
Histopathological types
|
0.332
|
0.059
|
0.780
|
0.229
|
0.372
|
0.176
|
|
Degree of dysplasia
|
0. 129
|
0. 181
|
0.080
|
0.051
|
0.052
|
0. 308
|
Table 7: Correlations of ER and PR immunohistochemical expression with different clinicopathological parameters in patients with colorectal carcinomas.
|
Parameter
|
Area (A)
(p value)
|
No. of objects (N)
(p value)
|
Intensity (I)
(p value)
|
|
ER
|
PR
|
ER
|
PR
|
ER
|
PR
|
|
Age
|
0.380
|
0.361
|
0.903
|
0.831
|
0.449
|
0.191
|
|
Gender
|
0. 490
|
0.705
|
0.190
|
0.140
|
0.204
|
0.353
|
|
Site
|
0.757
|
0.962
|
0.252
|
0.735
|
0.732
|
0.722
|
|
Size
|
0.832
|
0.648
|
0.619
|
0.988
|
0.949
|
0.781
|
|
Gross morphology
|
0.281
|
0.874
|
0.058
|
0.797
|
0.071
|
0.963
|
|
Histopathological types
|
0.142
|
0.603
|
0.108
|
0.732
|
0.285
|
0.645
|
|
Grade
|
0.280
|
0.473
|
0.304
|
0.593
|
0.256
|
0.485
|
|
Stage
|
0.249
|
0.853
|
0.056
|
0.691
|
0.072
|
0.813
|
|
Lymph node involvement
|
0.165
|
0.944
|
0.087
|
0.285
|
0.053
|
0.587
|
|
Lymphovascular permeation
|
0.253
|
0.544
|
0.837
|
0.337
|
0.156
|
0.406
|
|
Lymphocytic infiltration
|
0.814
|
0. 340
|
0.619
|
0. 337
|
0.782
|
0. 211
|
A
B
Figure 3: Correlations between ER and PR: (A) Significant positive correlation between ER and PR in adenoma (r=0.312. p=0.034). (B) Significant positive correlation between ER and PR in carcinoma (r=0.321, p=0.0398).
Discussion
It has recently been discovered that estrogen receptors (ER) and progesterone receptors (PR) are expressed both in normal mucosa, malignant colon and rectal tissues. As improvement in techniques of the detection technology and investigation deepened gradually, the role of ER and PR in tumorigenesis and development of colorectal cancer is becoming more evident.10 Recently, a study comparing estrogen-only HRT against placebo in women with previous hysterectomy revealed no difference in the prevalence of colorectal cancer (CRC) or survival after developing CRC. The authors postulated that progesterone protected against CRC by modulating the effects of estrogen on carcinogenesis.11
To the best of our knowledge, this is the first study conducted in Iraq concerning the role of ER and PR in colorectal carcinogenesis and showed that there is a gradual increase in ER and PR expression from control through adenoma to carcinoma in terms of frequency of positive cases and frequency of cases with strong intensity of staining, as well as the three digital parameters of immunohistochemical staining (A, N and I). The frequency of ER and PR expression in colorectal cancer was (42.42%, 36.36%, respectively), and there was a significant positive correlation of ER and PR in both adenoma and carcinoma. Concerning the clinicopathological parameters; the present work found a non-significant correlation of ER and PR immunohistochemical expression with the clinicopathological parameters studied. The data suggests that these receptors have some role in the process of colorectal carcinogenesis; however, their expression could not predict the risk of malignant transformation of premalignant lesions (adenoma) or the malignant biological behaviors of colorectal cancer.
Comparable studies, either biochemically or immuno-histochemically, revealed contradictory data. By biochemical methods; ER exhibited a wide range, varying from 20% to 54%, while PR expression exhibited around 42%.12-14 Immunohistochemically; some studies essentially showed no ER or PR positivity.4,15 Moreover, in one of the largest immunohistochemical studies in which 156 colonic carcinoma cases were examined; none were positive for ER, and only one case was reactive for PR.14 On the other hand, ER and PR reactions were consecutively found in 32% and 23% in colorectal carcinoma by Kaklamanos et al.16 Also, Wenxi et al. showed that ER was detected in 75.4% of colorectal carcinoma.17 Jun-Yan reported that positive ER and PR in colorectal cancer were observed in 59.3% and 65.5% of cases respectively and there was a statistically significant positive correlation between expressions of ER and PR, this finding is similar to the findings of the present study.18
In comparing clinicopathological significance of both receptors, some studies are in good accordance with the present study. Wenxi et al. investigated the immunohistological expression of ER in 65 patients with colorectal carcinoma and showed that the rates of positive expression of ER was significantly higher in tumor tissues than in normal mucosa (p=0.05). ER expression in the tumor was independent of sex and age of the patients, size, site and Dukes stage of the tumors.17 Zike et al. and Zike et al. also recorded that the immunohistochemical expression of ER in colorectal cancers was not related to patients’ age, sex, tumor position, pathological type, histological type, or stage.19,20 While Zhou et al. showed that ER and PR expression quantitatively measured using radioligand binding assay (RBA), in CRC tissues were higher than those in normal mucosa, and there was a positive correlation between ER and PR expression in tumor tissues. A correlation was detected between ER expression in tumor tissues and patients' age, but no correlation between tumor tissues PR expression and age was observed. Moreover, there was no correlation between tumor tissues ER, PR expression and sex, staging, tumor location, size, gross and histological type, invasive depth, or lymph nodes metastasis.21 Other studies that evaluated ER and PR have also shown that the percentages of ER or PR positive tumors are not different by tumor location or other tumor characteristics.12,22
In contrast, some studies are in discordance to the present study. Zavarhei et al. recorded for the first time that PR expression was significantly correlated with tumor size and stage, and that it was correlated with venous invasion in females rather than males.8 While Kaklamanos et al. found that the expression of receptors for sex steroids correlates with advanced stage disease. The expression of PR by the tumor cells is associated with a shorter patient survival.16 Furthermore, Qi et al. concluded that the combined expressions of ER, PR, p53 gene and DNA ploidy were correlated with better prognosis of colorectal cancer patients (p=0.01).23
Review of literature showed few rather old studies on the expression of ER and PR in colorectal adenomas. Marugo et al. found that ER and PR were expressed in colonic cancer, in colonic adenomas (so-called precancerous disease), as well as in normal mucosa; and suggested that the expression of steroid receptors could be considered as a marker of a precancerous condition.24 Concolino et al. reported that steroid receptors were usually found in the cytosol of the large polyps of male rather than female patients (46% vs. 11%) and in the cytosol of the adenomas with moderate or severe dysplasia in male patients. Malignant lesions usually possessed both ER and PR in the cytosol and nuclear fraction.25 Uehara et al. examined ER immunohistochemically in 37 specimens from colorectal cancer tissue and 28 specimens from adenomatous polyp tissue in the colon and rectum. Positive ER staining was observed in 30% of colorectal cancer tissue and 29% of adenomatous polyp tissue. Hence, no significant difference was observed in the incidence of positive ER staining according to sex. Cancer progression did not correlate with the incidence of positive staining. Tissue from the rectum has a tendency to show higher incidence of positive staining than that from the right-side of the colon. It was also noted that the more severe epithelial atypia in adenomatous polyp tissue had a tendency to show positive ER staining more frequently.26
Discrepancies in the results of studies could stem from several sources, including the source of tissue, methods of detecting ER and PR, and level of staining at which ERs and PRs are declared positive. Many studies have relied on fresh tissue, while in the present study; tissue was obtained from paraffin blocks. Different techniques to detect ER and PR have also been used by different studies. In earlier studies using the Dextran-coated charcoal (DCC) assay, high levels of ER and PR proteins in the cytosolic and nuclear fractions of both normal and malignant mucosa were detected.24 Subsequent studies testing fresh and paraffinized specimens by using a more specific monoclonal antibody-based immunoassay demonstrated low levels of ER and PR.27,28
The antibodies used in the present study are used on a clinical basis to detect ER and PR in breast tissue, and have been shown to be effective in detecting ER- and PR-positive tumors using paraffin-fixed blocks. It may be possible that using other antibodies or techniques could yield different results. However, compared with the manual immunohistochemical method; there are major benefits of using an image analyzer (Digimizer) to quantify immunohistochemical staining. First; it converts a qualitative or semiquantitative assay into a truly quantitative assay. Second; it can improve assay objectivity and reproducibility. Third; such image analyzer-assisted immunohistochemical quantification does not require much additional training and is simple to perform. Instead of adding a totally new assay to laboratory operation, it modifies only the quantitation step while keeping the previous immunohistochemical assay steps unchanged. However, the implementation of the Automated Cellular Imaging System (ACIS) is likely to be an expensive process. In addition, the cost of continuous use and maintenance of such an image analyzer may be substantial. Software and hardware upgrades also may be needed.29
Conclusion
In summary, ER and PR expression was increased in a sequence of; normal colonic mucosa-adenoma-carcinoma, and there was a positive correlation between ER and PR expression in adenoma and carcinoma indicating that ER and PR may play a role in colorectal carcinogenesis. Further studies are recommended to determine the role of ER, PR and their isomers in the genesis, physiology, pathology and pharmacology of the colon and rectum, which may be promising to provide new ideas for both therapy and prognostic evaluation of colorectal cancer.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Elledge RM, Fuqua SA. Estrogen and Progesterone Receptors. Available at:http://drugswell.com/wowo/blog1.php/2011/01/16/estrogen-and-progesterone-receptors. Accessed at March 2011.
2. Cleveland AG, Oikarinen SI, Bynoté KK, Marttinen M, Rafter JJ, Gustafsson JA, et al. Disruption of estrogen receptor signaling enhances intestinal neoplasia in Apc(Min/+) mice. Carcinogenesis 2009 Sep;30(9):1581-1590.
3. Cho NL, Javid SH, Carothers AM, Redston M, Bertagnolli MM. Estrogen receptors α and β are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res 2007 Mar;67(5):2366-2372.
4. Ekem TE, Bahadir B, Gun BD, Bektas S, Kertis G, Yurdakan G, et al. Colorectal carcinomas: Clinicopathologic investigation, correlation with expression of estrogen and progesterone receptors. Turkish Journal of Cancer 2008;38:118-122.
5. Jiang HP, Teng RY, Wang Q, Zhang X, Wang HH, Cao J, et al. Estrogen receptor alpha variant ERalpha46 mediates growth inhibition and apoptosis of human HT-29 colon adenocarcinoma cells in the presence of 17beta-oestradiol. Chin Med J (Engl) 2008 Jun;121(11):1025-1031.
6. Grodstein F, Martinez ME, Platz EA, Giovannucci E, Colditz GA, Kautzky M, et al. Postmenopausal hormone use and risk for colorectal cancer and adenoma. Ann Intern Med 1998 May;128(9):705-712.
7. Schindler AE. Long-term use of progestogens: colon adenoma and colon carcinoma. Gynecol Endocrinol 2007 Oct;23(Suppl 1):42-44.
8. Zavarhei MD, Bidgoli SA, Ziyarani MM, Shariatpanahi M, Ardalan FA. Progesterone receptor positive colorectal tumors have lower thymidine phosphorylase expression: an immunohistochemical study. Pak J Biol Sci 2007 Dec;10(24):4485-4489.
9. Qasim ZJ. Co-expression of (VEGF A, VEGF C, COX2and EGFR) biomarkers in human colorectal cancer and their association with lymph node metastasis and angiogenesis. A Thesis Submitted to College of Medicine -Al-Nahrain University2010.
10. Bing Z, Chuan-min Z. Progress of relationship between ER, PR and prognosis of colorectal cancer. Medical Recapitulate 2008;21:14-20.
11. Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol 2010 Jan;25(1):33-42.
12. Meggouh F, Lointier P, Pezet D, Saez S. Status of sex steroid hormone receptors in large bowel cancer. Cancer 1991 Apr;67(7):1964-1970.
13. Di Leo A, Messa C, Russo F, Misciagna G, Guerra V, Taveri R, et al. Prognostic value of cytosolic estrogen receptors in human colorectal carcinoma and surrounding mucosa. Preliminary results. Dig Dis Sci 1994 Sep;39(9):2038-2042.
14. Slattery ML, Samowitz WS, Holden JA. Estrogen and progesterone receptors in colon tumors. Am J Clin Pathol 2000 Mar;113(3):364-368.
15. Cameron BL, Butler JA, Rutgers J, Vargas HI, Purtell M, Sheppard B. Immunohistochemical determination of the estrogen receptor content of gastrointestinal adenocarcinomas. Am Surg 1992 Dec;58(12):758-760.
16. Kaklamanos IG, Bathe OF, Franceschi D, Lazaris AC, Davaris P, Glinatsis M, et al. Expression of receptors for estrogen and progesterone in malignant colonic mucosa as a prognostic factor for patient survival. J Surg Oncol 1999 Dec;72(4):225-229.
17. Wenxi W, Lizong S, Dongming Y, et al. Expression of estrogen receptor and P glycoprotein in primary colorectal carcinoma. The Practical Journal of Cancer 1999;03:20-28.
18. Jun-Yan L. Receptors of estrogen and progesterone in human colorectal carcinoma: detection and their significance in the prognosis. Practical Clinical Medicine 2002;03:16-20.
19. Zike Q, Desen W, Junyan L, et al. Expression of nm23 protein and estrogen receptor and prognosis of colorectal cancers. Chinese Journal of Surgery 2000;07:12-16.
20. Zike Q, Desen W, Jun-yan L, Han-liang L, Jing-hui H. Expression and prognostic values of p21 protein and estrogen receptor in colorectal cancers. Chinese Journal of Cancer 2001;20:5-9.
21. Zhou ZW, Wan DS, Wang GQ, Pan ZZ, Lu HP, Gao JH, et al. Expression of estrogen receptor and progesterone receptor in colorectal cancer: a quantitative study. Ai Zheng 2004 Jul;23(7):851-854.
22. Meggouh F, Lointier P, Saez S. Sex steroid and 1,25-dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normal mucosa. Cancer Res 1991 Feb;51(4):1227-1233.
23. Qi L, Sonen Z, Shifeng Z. The detection of ER, PR and DNA ploidy,p53 gene in colorectal cancer and its clinical value. Journal of Chongqing Medical University 2009;08:32-37.
24. Marugo M, Molinari F, Fazzuoli L, Parodi MC, Bernasconi D, Menozzi F, et al. Estradiol and progesterone receptors in normal and pathologic colonic mucosa in humans. J Endocrinol Invest 1985 Apr;8(2):117-119.
25. Concolino G, Arrabito G, Buonomo O, Paesani P, Conti C, Picardi C. Nuclear steroid receptors and dysplasia in adenomatous polyps of the colon as markers of high risk for malignant transformation. Cancer Detect Prev 1986;9(5-6):477-484.
26. Uehara Y, Kojima O, Takemoto Y, Majima T, Takahashi T, Tada M. Immunohistochemical study of endogenous estrogen in colorectal cancer and adenomatous polyp tissue. Dig Surg 1986;3:236-239 .
27. Galandiuk S, Miseljic S, Yang AR, Early M, McCoy MD, Wittliff JL. Expression of hormone receptors, cathepsin D, and HER-2/neu oncoprotein in normal colon and colonic disease. Arch Surg 1993 Jun;128(6):637-642.
28. Hendrickse CW, Jones CE, Donovan IA, Neoptolemos JP, Baker PR. Oestrogen and progesterone receptors in colorectal cancer and human colonic cancer cell lines. Br J Surg 1993 May;80(5):636-640.
29. Wang S, Saboorian MH, Frenkel EP, Haley BB, Siddiqui MT, Gokaslan S, et al. Assessment of HER-2/neu status in breast cancer. Automated Cellular Imaging System (ACIS)-assisted quantitation of immunohistochemical assay achieves high accuracy in comparison with fluorescence in situ hybridization assay as the standard. Am J Clin Pathol 2001 Oct;116(4):495-503.
|