Metabolic syndrome (MetS) is a condition associated with an increase in various risk components1 that predisposes an individual to both cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM).2 It is a complex condition, which has now become a worldwide epidemic. The components of MetS include abdominal obesity, insulin resistance (IR), dyslipidemia (increased triglycerides (TG), decreased high-density lipoprotein cholesterol (HDL-c), and increased low density lipoproteins cholesterol (LDL-c) and elevated blood pressure (BP).3 MetS is associated with an up to five-times increased risk of T2DM and an approximately two-fold increased risk of CVD.4 This is due to clustering of various risk factors in patients with MetS. Population-based cohort studies on subjects without CVD and diabetes mellitus (DM) at presentation have demonstrated that the mortality rates due to these conditions were greater in those with MetS.5
To date, the exact pathogenesis of the MetS is unknown; however, increased abdominal adiposity and IR are considered to play a central role. Excess adipose tissue releases a number of proinflammatory factors that cause low-grade inflammation, thus rendering MetS an inflammatory state.6 Inflammation also plays role in the development of diabetes and atherosclerosis7 and markers of inflammation can help in risk prediction for CVD and DM. Of the various inflammatory markers studied, evidence suggests that high-sensitivity C reactive protein (hs-CRP) is the most useful.8 Thus, it can be hypothesized that inflammation is the underlying abnormality linking MetS to DM and CVD, and the study of inflammatory marker levels like hs-CRP in MetS could be useful for risk identification.
We sought to determine the levels of inflammatory marker hs-CRP in MetS subjects and its correlation with various MetS components.
Methods
We performed a case-control study in the Chemical Pathology department of the Army Medical College, National University of Sciences and Technology, Rawalpindi, Pakistan, over a period of six months (from January to June 2013). Study approval was obtained by the institutions ethical committee and informed consent was obtained from all patients.
A total of 200 patients with MetS (n=100) along with age and gender matched controls (n=100). The patients were evaluated both clinically and by laboratory investigations. MetS was defined by the modified International Diabetes Federation (IDF) criterion [Box 1] with ethnic specific cut offs for Asians. The presence of abdominal adiposity is an essential criterion along with the presence of at least two other criteria in order to define MetS. The units were converted to system of international units (SI) for standardization.
Box 1: Metabolic Syndrome (MetS) as defined by the modified International Diabetes Federation criterion. The presence of abdominal obesity and two other criteria determines the presence of MetS.
|
Abdominal obesity (cm): males >90, females >80 |
|
Dysglycemia: fasting plasma glucose (mmol/L) ≥5.6 |
|
Hypertension (mmHg): ≥130/≥85 |
|
High triglycerides (mmol/L): ≥1.70 |
Subjects with MetS without any preexisting CVD, hematological, neoplastic, renal, liver, or thyroid disease were included in the study. Patients with infectious or autoimmune diseases, familial hyperlipidemia, inflammatory diseases, smokers, pregnant ladies, patients taking lipid lowering medication, and those unable to give informed consent were excluded.
Healthy subjects were used as controls. Those with any acute or chronic illness or those on anti-inflammatory drugs were excluded along with smokers, pregnant women, and women on oral contraceptives pills.
To rule out inflammation, complete blood count (CBC), erythrocyte sedimentation rates (ESR), and serum alanine aminotransaminase (ALT) biochemical tests were performed. Patients with leucocytosis, lymphocytosis, raised ESR and/or serum ALT raised more than twice its upper reference limit (42U/L), and those with hs-CRP ≥10mg/L were also excluded.
At the time of presentation demographic details were recorded and complete history was obtained regarding the presence of CVD, DM, hypertension, any inflammatory disease, joint pains, infections, hyperlipidemias, endocrine disorders, allergies, and smoking habits. Drug history was also obtained. The details were noted on a questionnaire.
Anthropometric measurements were recorded to calculate body mass index (BMI). Weight (in kg) was taken on a standardized weighing scale with light clothing and bare feet and given to the nearest 0.1kg. Height (in m2) was measured on a standardized chart. Waist circumference (WC) and hip circumference (HC) were measured with a non-stretchable measuring tape for obtaining waist to hip ratio (WHR). WC was measured at the midpoint between the anterior superior iliac spine and the lowest rib. HC was measured at the most protuberant part of gluteus maximus. The WHR was defined as the ratio of waist girth to the circumference of the hips. BP was measured after the patient had been seated for 10 minutes.
About 10ml of blood was obtained in fasting state (duration of fast 12 hours) and was divided into plain tubes for serum analysis and plasma tubes containing sodium potassium EDTA. The patient’s lipid profile, hs-CRP, and insulin levels were estimated in serum while glucose was measured in plasma. Glucose, TG, and HDL-c were analyzed immediately after centrifugation and the samples were then stored at -20oC until analysis of hs-CRP and insulin were performed. Blood glucose, TG, and HDL-c were measured by enzymatic colorimetric methods using an automated clinical chemistry analyzer (Selectra E; Merck, Netherlands). Serum hs-CRP and insulin were analyzed using the Immulite 1000 (Diagnostic Product Corporation, USA).
IR was calculated by the homeostasis mode assessment insulin resistance (HOMA-IR) using the following formula:
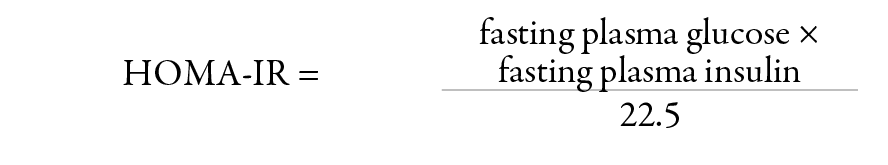
The data was analyzed using SPSS version 17. For numerical data mean and standard deviation (SD) were calculated. Categorical data was compared using the chi-square test and the independent t-test was employed for comparison of means between the case and control groups. Correlation analysis of hs-CRP with the MetS features was performed using Spearman’s correlation. Linear regression analysis with inflammatory marker hs-CRP as the dependent variable and other clinical and biochemical determinants of MetS as predictors was performed. Statistical significance was established at p<0.050.
Results
A total of 100 patients with MetS were included with an equal number of age and gender matched controls. The mean age of the patients with MetS was 51±11 years and of the control group was 40±12 years. The MetS group was made up of 31% males and 69% females, and the control group was 28% male and 72% female.
Patients with MetS were more obese, as depicted by increased BMI, WC and WHR. The cases represented a state of IR by elevated fasting insulin levels and HOMA-IR values. The value of HOMA-IR <2 was considered normal, a value ≥2 was pathologic, and values >4 represented the prediabetic phase.10 HOMA-IR in our subjects was 3±1 implying a pathological to prediabetic stage. MetS subjects also exhibited a dyslipidemic profile with raised TG and decreased HDL-c.
The clinical and biochemical features of patients and controls are shown in Table 1. hs-CRP (mg/L) was elevated in MetS patients (3±2) compared to the control group (0.5±0.3, p<0.010) as shown in Figure 1.
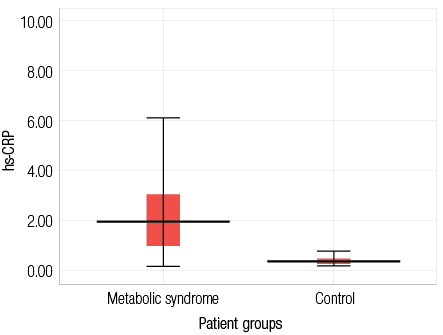
Figure 1: Serum high sensitive C reactive protein (mg/L) in patients with metabolic syndrome and the control group. Data are presented as mean±SD.
Table 1: Clinical and biochemical features of patients with metabolic syndrome and the control group.
|
BMI, kg/m2 |
37±7 |
25±5 |
<0.010 |
|
WC, cm |
110±14 |
86±20 |
<0.010 |
|
WHR |
1.03±0.08 |
0.86±0.09 |
<0.010 |
|
Fasting glucose, mmol/L |
8.81±3.65 |
6.31±2.99 |
<0.010 |
|
Fasting insulin, mIU/L |
9.01±7.35 |
3.99±3.22 |
<0.010 |
|
HOMA-IR |
3±1 |
1±1 |
<0.010 |
|
Triglyceride, mmol/L |
2.22±0.64 |
1.58±0.95 |
<0.010 |
|
HDL-c, mmol/L |
0.94±0.26 |
1.13±0.31 |
<0.050 |
|
BPS, mmHg |
125±13 |
115±16 |
<0.010 |
BMI: body mass index; WC: waist circumference; WHR: waist to hip ratio; HOMA-IR: homeostasis mode assessment insulin resistance; HDL-c: high-density lipoprotein cholesterol; BPS: blood pressure systolic; and BPD: blood pressure diastolic
A significant correlation was established between
MetS components and hs-CRP, with the exception of systolic and diastolic blood pressure. Linear regression analysis performed with hs-CRP as the dependent variable and clinical and biochemical features of MetS as independent variables revealed that the only significant predictors of hs-CRP were WC, fasting insulin levels, and HOMA-IR.
Discussion
In our study, we found that hs-CRP was significantly raised in MetS patients compared to the control group, which is in accordance with many studies conducted previously.11-13 This can be explained by the fact that inflammation causes metabolic dysfunction.14 Systemic inflammatory activity also plays role in atherogenesis and IR leading to CVD and T2DM.13 Thus low grade inflammation is associated with both the pathogenesis of MetS and its associated complications.15 We have also demonstrated that WC, fasting insulin, and HOMA-IR were the main predictors of hs-CRP. This is in accordance with the study by Zuliani G et al,16 which suggested that IR and visceral adiposity, as measured by WC, gave rise to low grade inflammation. WC is strongly related to visceral fat and abdominal adiposity,17 which plays central role in MetS.15 Visceral adipose tissue has number of metabolic, endocrine, and immunological functions that are intricately linked to the pathogenesis of the MetS.18 This is achieved through secretion of a variety of cytokines or adipokines. Altered secretion of these lead to inflammation along with alterations in glucose and lipid homeostasis.19 This has in turn been linked to nutrient excess, which causes adipocyte hypertrophy and hyperplasia inciting hypoxic injury that triggers the inflammatory response.20 How this inflammation causes metabolic dysfunction is not completely understood; however, a number of molecular signaling pathways are considered to be involved in directing a metabolic challenge into an inflammatory response,14 such as inhibition of the insulin receptor cascade and endothelial dysfunction.20 In our study, hs-CRP was also found to correlate positively with IR suggesting role of inflammation between IR and development of DM.21
Therefore, MetS can be viewed as a result of complex interaction between obesity and IR.22 This view is also supported by our study and our results suggest that inflammation in MetS represents a synergy between adiposity and IR. Inflammation is the common unifying link between conditions like CVD and DM.20 hs-CRP has been established as an inflammatory marker having a positive predictive value for future CVD and diabetes.11 hs-CRP levels are stratified into low risk (<1), moderate risk (1 to <3), and high risk (≥3mg/L) categories.23 Adding hs-CRP to the risk assessment of people without CVD could be used as a predictor of a future CVD event.24 The mean hs-CRP in patients with MetS in our study was 2.53±2.07mg/L. This implies that these individuals might be the victims of ongoing silent atherosclerotic changes predisposing them to CVD. It is suggested that hs-CRP should be added to MetS screening protocols where it can also be used to monitor preventive therapies against metabolic disorders like DM and CVD. However, large scale prospective studies are required in this regard particularly in underdeveloped countries. Early identification can save not only treatment costs but can also help in devising prevention strategies.
Conclusion
MetS results from the interaction between visceral adiposity and IR leading to a state of chronic low grade inflammation. Patients with MetS thus represent a group whom primary prevention therapies should be targeted before it culminates in overt CVD or DM. Large multicentric studies are needed to gain insight into its pathogenesis and derive treatment strategies.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- Hoebel S, Malan L, De Ridder H. Differences in MetS marker prevalence between black African and Caucasian teachers from the North West Province: Sympathetic activity and ambulatory blood pressure in Africans (SABPA) Study. Journal of Endocrinology, Metabolism and diabetes South Africa 2011; 16(1):49-56.
- Rice BH, Cifelli CJ, Pikosky MA, Miller GD. Dairy components and risk factors for cardiometabolic syndrome: recent evidence and opportunities for future research. Adv Nutr 2011 Sep;2(5):396-407.
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med 2011 May;9:48.
- Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2012 Feb;59(7):635-643.
- Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 2009 Spring;4(2):113-119.
- Eisenbarth GS, ed. Immunoendocrinology: scientific and clinical aspects. Springer Science & Business Media 2010.
- Gomes MD. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens 2013 Mar;2013:653789.
- Pfützner A, Schöndorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol 2010 May;4(3):706-716.
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasim JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Ass Physicians India 2009 Feb; 57:163-170.
- Sink A. Insulin resistance in patients with chronic hepatitis C. TMJ 2007;57(4):240-244.
- Vidyasagar S, Abdul Razak UK, Prashanth CK, Muralidhar Varma D, Bairy KL. Highly Sensitive C - Reactive Protein in metabolic syndrome. Journal, Indian Academy of Clinical Medicine 2013;14(3-4):230-234.
- Pravenec M, Kajiya T, Zídek V, Landa V, Mlejnek P, Simáková M, et al. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension 2011 Apr;57(4):731-737.
- Abu-Farha M, Behbehani K, Elkum N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc Diabetol 2014 Apr;13(76):76.
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011 Jun;121(6):2111-2117.
- Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;1-10.
- Zuliani G, Volpato S, Galvani M, Blè A, Bandinelli S, Corsi AM, et al. Elevated C-reactive protein levels and metabolic syndrome in the elderly: The role of central obesity data from the InChianti study. Atherosclerosis 2009 Apr;203(2):626-632.
- Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol 2014;2014:1-7.
- Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 2012 Feb;71(1):181-189.
- Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013 Jan;93(1):359-404.
- Emanuela F, Grazia M. Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab 2012; 2012(1):1-7.
- Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr 2014 Sept;6(1):99.
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 2008 Apr;88(2):389-419.
- Oda E, Kawai R. Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern Med 2010;49(2):117-124.
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al; Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012 Oct;367(14):1310-1320.