Epilepsy is a common neurological disorder with a median lifetime prevalence of 14 per 1000 subjects in developing countries.1 The discrete electroclinical event, termed epileptic seizure, could be influenced by changes in the physiological sleep pattern. In certain epileptic syndromes, for example nocturnal frontal lobe epilepsy, seizures are typically encountered during sleep. In general, sleep disorders are also highly prevalent in the general population. However, only in recent years considerable attention has been focused on the comorbid sleep disorders in patients with epilepsy.2,3
Among the sleep disorders, obstructive sleep apnea syndrome (OSAS) is a common problem occurring in 2% of adult women and 4% of adult men in the general population.4 OSAS disrupts sleep and can cause significant sleep deprivation and fragmentation, causing impaired sleep continuity, increased daytime sleepiness and impaired cognitive functions.5 Such alterations in sleep pattern could have considerable influence on seizure activity in patients with epilepsy. The frequency of comorbid OSAS among patients with epilepsy and the relationship between the severity of epilepsy and the degree of obstruction in OSAS are still not well studied. We conducted this study to estimate the hospital-based frequency of OSAS among patients with epilepsy and to study the seizure characteristics among those patients
with comorbid OSAS.
Method
We recruited patients with a confirmed diagnosis of epilepsy attending the neurology clinic of the Sultan Qaboos University Hospital, Muscat, Oman, between June 2011 and April 2012. We included adult patients above the age of 18 years. The diagnosis of epileptic seizure was based on clinical characteristics with or without electrographic ictal abnormalities. Those with purely psychogenic seizures, uncertain diagnosis (for instance patients with a differential diagnosis of seizure vs. syncope), symptomatic or provoked seizures (seizure occurring as a symptom or manifestation of a known cerebral insult) and those who were diagnosed with epilepsy but lost to follow-up before the study period were excluded. The study was approved by the institutional ethics committee.
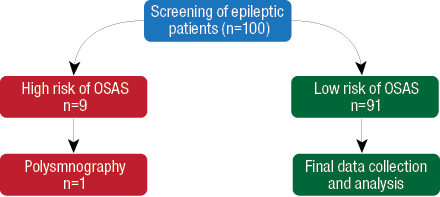
Figure 1: Flow chart of the patients included in the cross-sectional retrospective study.
In this cross-sectional retrospective study, patients with epilepsy were screened for OSAS by direct interview using the validated Arabic version of the Berlin questionnaire.6,7 Those who scored high on the questionnaire underwent diagnostic polysomnography. The flow of patients is shown in Figure 1.
Each patient completed clinical evaluation including appropriate medical history and clinical examination, and the Epworth sleepiness scale. They also underwent a full diagnostic polysomnography (Grass, Astromed, USA). The polysomnography was conducted and scored according to American Academy of Sleep Medicine (AASM) guidelines8 and has been described fully in our previous studies.9,10
Descriptive statistics in the form of percentage, mean with standard deviation (SD), and range were used.
Results
Table 1: Clinical characteristics of cohort of patients with epilepsy.
|
Gender |
|
|
Male |
55 |
|
Female |
45 |
|
Age range(14-58 years) |
|
|
<20 |
24 |
|
20-40 |
61 |
|
>40 |
15 |
|
Mean |
28 |
|
Median |
27 |
|
Body mass index (n=59)* |
27 ±7 |
|
Range |
16–47 |
|
Comorbid medical conditions (Median) |
25.2 |
|
Hypertension |
8 |
|
Diabetes |
4 |
|
Type of Epilepsy |
|
|
Generalized |
67 |
|
Focal |
27 |
|
Unclassified |
6 |
|
Seizure frequency |
|
|
Almost everyday |
9 |
|
1–2 times per week |
6 |
|
1–2 times per month |
19 |
|
Never or almost never |
65 |
* Mean ±SD
One hundred patients with epilepsy (55 male and 45 female) were screened for OSAS. The clinical characteristics of these subjects are shown in Table 1.
Generalized seizure was found in 67% of patients (n=36 male, n=31 female), 27% had focal seizure (n=17 male, n=10 female) and 6% of the patients had epilepsy of an undetermined type. The majority of our patients had low frequency of seizures. Sixty five percent of patients had almost no seizure attacks for a minimum of six months at the time of screening, 19% had one to two attacks per month, 6% had one to two seizures per week, and 9% had seizures almost every day.
The average epilepsy duration in our sample was 13 years (SD±9 years). The majority (62.2%) of patients had been diagnosed with epilepsy for more than 10 years, 27% for less than five years, and 10% between five and10 years [Table 1].
Table 2: Anti-epileptic drug use and risk of obstructive sleep apnea syndrome (OSAS).
|
Risk |
|
Low |
50 (92.6) |
28 (87.5) |
11 (91.7) |
89 (90.8) |
|
High |
4 (7.4) |
4 (12.5) |
1 (8.3) |
9 (9.2) |
|
Total |
54 (100) |
32 (100) |
12 (100) |
98 (100) |
|
Daytime sleepiness |
|
Negative |
52 (96.3) |
28 (87.5) |
10 (83.3) |
90 (91.8) |
|
Positive |
2 (3.7) |
4 (12.5) |
2 (16.7) |
8 (8.2) |
|
Total |
54 (100) |
32 (100) |
12 (100) |
98 (100) |
|
Apnea |
|
Nearly everyday |
0 (0) |
1 (3.1) |
0 (0) |
1 (1) |
|
3–4 times a week |
2 (3.7) |
0 (0) |
2 (16.7) |
4 (4.1) |
|
1–2 times a week |
2 (3.7) |
0 (0) |
1 (8.3) |
3 (3.1) |
|
1–2 times per month |
7 (13) |
5 (15.6) |
1 (8.3) |
13 (13.3) |
|
Never |
43 (79.6) |
26 (81.3) |
8 (66.7) |
77 (78.6) |
Table 3: Subjects response to Berlin questionnaire.
|
Do you snore? |
| |
a. Yes |
41 |
| |
b. No |
59 |
| |
c. Don’t know |
0 |
|
Your snoring is: |
| |
a. Slightly louder than breathing |
25 |
| |
b. As loud as talking |
11 |
| |
c. Louder than talking |
4 |
| |
d. Very loud – can be heard in adjacent rooms |
1 |
|
How often do you snore? |
| |
a. Nearly everyday |
8 |
| |
b. 3–4 times a week |
3 |
| |
c. 1–2 times a week |
23 |
| |
d. 1–2 times a month |
7 |
| |
e. Never or nearly never |
0 |
|
Has your snoring ever bothered other people? |
| |
a. Yes |
9 |
| |
b. No |
27 |
| |
c. Don’t know |
5 |
|
Has anyone noticed that you quit breathing
during your sleep? |
| |
a. Nearly everyday |
1 |
| |
b. 3–4 times a week |
4 |
| |
c. 1–2 times a week |
3 |
| |
d. 1–2 times a month |
13 |
The analysis of Berlin Questionnaire determined that 41 of the 100 patients were snorers (26 male and 15 female). Over half of snorers (n=25) reported snoring slightly louder than breathing. Only one patient had a very loud snoring, which could be heard from the adjacent room. Twenty-three patients reported snoring one to two times a week and eight patients reported snoring every day. The rest of Berlin questionnaire results can be found in Tables 2 and 3. Hypertension was reported in eight patients. There was no significant association between generalized and focal epileptic patients and daytime sleepiness (p=0.700). However, positive daytime sleepiness was reported by only nine patients (9%). After analyzing patient’s responses to the Berlin questionnaire, 9% of the patients (n=9) were determined to be at high risk of developing OSAS. Only six patients with high risk OSAS had their BMI calculated (mean ±SD=34.20±7.21) and 53 patients with low risk OSAS (mean ±SD=26.06±5.86). A high BMI was significantly associated with the risk of OSAS (p= 0.003). No significant association was found with age (p=0.400) [Table 4]. The number of men and women in the high-risk group was nearly equal (four men and five women). Six patients with high risk of OSAS had generalized epilepsy. Four of the epileptic patients with high risk of OSAS received monotherapy, four received dual therapy, and only one received polytherapy [Table 2]. Twenty-six patients received sodium valproate and there was no significant association with snoring, breathing pauses and daytime sleepiness (p>0.100).
Discussion
This cross-sectional study aimed to find out the frequency of OSAS among patients with epilepsy using simple methodology, the Berlin questionnaire. The Berlin questionnaire is a valid tool to screen for OSAS6 in a source-limited setting and has been validated for Arabic speaking population.7 We included only adult patients (>18 years old) and that may have contributed to the limited number of patients below the age of 20 (24%). Furthermore, more than 50% of our patients had epilepsy for more than 10 years and the other 25% had it for less than five years. This could be related to the fact that most of the patients were 20–40 years old and had developed seizures in early childhood.
The study showed that the risk of OSAS was marginally greater in patients with epilepsy compared to general population4 with an overall prevalence of 9%. BMI revealed that 32% of patients were obese or overweight. BMI was calculated for only 59 patients due to incomplete data. However, another study reported obesity and overweight in 50% of patients with epilepsy.11 One possible explanation for patients with epilepsy being overweight is the type of anti-epileptic drugs (AED) the patient is taking, especially sodium valproate.11 However, we did not have enough evidence to confirm this explanation in our study. Snoring was reported in 41% of the epileptics, which was not not very different from general population.12 Daytime sleepiness was reported in only nine patients with no significant association with either type or severity of epilepsy nor with the number of AEDs. Other studies tried to evaluate the rate and features of OSAS in adult epilepsy patients. Manni et al, 13 found that the major risk factors for OSAS in epilepsy patients were the same as those typically found in the general population. Of the epilepsy-related factors, older age at onset of seizures appeared to be significantly related to comorbidity. He also found increasing evidence that OSAS coexists in epilepsy in 10% of unselected adult epilepsy patients and 20% of children with epilepsy, and up to 30% of drug-resistant epilepsy patients.14 However, in our study, we found no significant correlation of the presence of OSAS with the number or type of AEDs or duration of treatment.
The major limitations in this study were the small sample size and methodology. A larger sample size may give a better understanding of the real association between epilepsy and OSAS. Even though the Berlin questionnaire is a validated tool to assess high risk,7 the sensitivity of this method may not be as accurate as performing polysmnography. The inherent problem with the questionnaire is the results prone to subjective estimation and under-reporting of some parameters. Nevertheless, eight patients who had a high risk of OSAS refused or did not come to do the test and only one of them did polysmnography, which was not enough for statistical analysis. Other issues in the study included the unavailability of data related to height and weight in some of the patients’ records, therefore, we were unable to calculate the BMI for large number of patients (n=41).
Table 4: Obstructive sleep apnea syndrome (OSAS) risk factor in the study population.
|
Age |
91 |
27.8±9.8 |
9 |
20.89±10.093 |
0.367 |
|
Weight |
91 |
67.29±16.63 |
9 |
94.48±31.28 |
0.032** |
** Significant p-value <0.050
Conclusion
The study revealed that the frequency of OSAS among epilepsy patients is 9%. However, because of the limitations, a prospective study to screen bigger population of patients with epilepsy using portable sleep study, including airflow sensors, chest movement, body position, and finger oxygen saturation would be more reliable to estimate the real prevalence of sleep-related breathing disorder. This method is widely used in sleep medicine epidemiological and clinical studies.15
Disclosure
The authors declared no conflict of interest. No funding was received for this work.
references
- Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet 2012 Sep;380(9848):1193-1201. PubMed doi:10.1016/S0140-6736(12)61381-6.
- Derry CP, Duncan S. Sleep and epilepsy. Epilepsy Behav 2013 Mar;26(3):394-404. PubMed doi:10.1016/j.yebeh.2012.10.033.
- Ehrenberg B. Importance of sleep restoration in co-morbid disease: effect of anticonvulsants. Neurology 2000;54(5)(Suppl 1):S33-S37.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993 Apr;328(17):1230-1235.
- Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep 2000 Jun;23(23)(Suppl 4):S102-S108.
- Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 2008 May;108(5):822-830.
- Saleh AB, Ahmad MA, Awadalla NJ. Development of Arabic version of Berlin questionnaire to identify obstructive sleep apnea at risk patients. Ann Thorac Med 2011 Oct;6(4):212-216.
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester. American Academy of Sleep Medicine. 2007;IL:45-47.
- Al-Abri M, Al-Hashmi K, Jaju D, Al-Rawas O, Al-Riyami B, Hassan M. Gender difference in relationship of apnoea/hypopnoea index with body mass index and age in the omani population. Sultan Qaboos Univ Med J 2011 Aug;11(3):363-368.
- Al-Abri M, Al-Hamhami A, Al-Nabhani H, Al-Zakwani I. Validation of the Arabic version of the Epworth sleepiness scale in Oman. Oman Med J 2013 Nov;28(6):454-456.
- Morrell MJ, Isojärvi J, Taylor AE, Dam M, Ayala R, Gomez G, et al. Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Res 2003 May;54(2-3):189-199.
- Ohayon MM, Guilleminault C, Priest RG, Caulet M. Snoring and breathing pauses during sleep: telephone interview survey of a United Kingdom population sample. BMJ 1997 Mar;314(7084):860-863.
- Manni R, Terzaghi M, Arbasino C, Sartori I, Galimberti CA, Tartara A. Obstructive sleep apnea in a clinical series of adult epilepsy patients: frequency and features of the comorbidity. Epilepsia 2003 Jun;44(6):836-840.
- Manni R, Terzaghi M. Comorbidity between epilepsy and sleep disorders. Epilepsy Res 2010 Aug;90(3):171-177.
- Punjabi NM, Aurora RN, Patil SP. Home sleep testing for obstructive sleep apnea: one night is enough! Chest 2013 Feb;143(2):291-294.