|
Abstract
Solid pseudopapillary neoplasm of the pancreas is a rare tumor of the pancreas often detected initially on imaging. Of uncertain histogenesis, it has a low-grade malignant potential with excellent post-surgical curative rates and rare metastasis. Despite advances in imaging, pseudocysts and other cystic neoplasms feature in the differential diagnosis. Pathological and/or cytological evaluation remains the gold standard in reaching a definitive diagnosis. On morphology alone, other primary pancreatic tumors and metastatic tumors pose a diagnostic challenge. Recent advances in immunohistochemical characterization have made the histopathologic diagnosis more specific and, in turn, shed light on the likely histogenesis of this rare tumor. We report a case of solid pseudopapillary neoplasm of the pancreas that was suspected on radiology and diagnosed intraoperatively on imprint cytology guiding definitive surgery. The diagnostic dilemmas are reviewed.
Keywords: Solid pseudopapillary neoplasm (SPN); Pancreas; Beta-catenin; E-cadherin.
Introduction
Solid pseudopapillary neoplasm of the pancreas is a rare cystic neoplasm. Its unique characteristics include its occurrence in young females, uncertain histogenesis, unpredictable malignant potential, as well as its excellent long term prognosis, even when it appears to be aggressive at presentation.1,2 Accurate diagnosis aids surgical decision making and, where necessary, multimodality management.3-5
We report a case of SPN in which diagnosis was suspected on radiology and confirmed intra-operatively by cytology, enabling appropriate surgical management. The diagnostic dilemmas and current knowledge of the origins, behavior, and treatment options of this neoplasm are presented.
Case Report
A 23-year-old Omani female presented with gradually progressing, episodic, epigastric pain radiating to the right hypochondrium with recent accentuation. Pain was accompanied by anorexia and significant weight loss (5 kg in one month). There was no relationship to food or bowel movements. Her mother had died of metastatic gastric carcinoma. General and systemic examination revealed no abnormality. Of note, no mass could be palpated in the abdomen. Hematologic and metabolic parameters, including tumor markers were within normal limits.
A CT scan of the abdomen and pelvis showed a 7.7 × 7.5 × 7 cm enhancing, rounded, well-defined cystic and solid lesion, located supero-anterior to the left kidney (Figs. 1A and B). The pancreatic tail was splayed and poorly delineated. The possibility of an epithelial solid and cystic pancreatic mass or a gastrointestinal stromal tumor (GIST) was entertained. A proposed endoscopic ultrasound and fine needle aspiration cytology of the mass was declined by the patient.
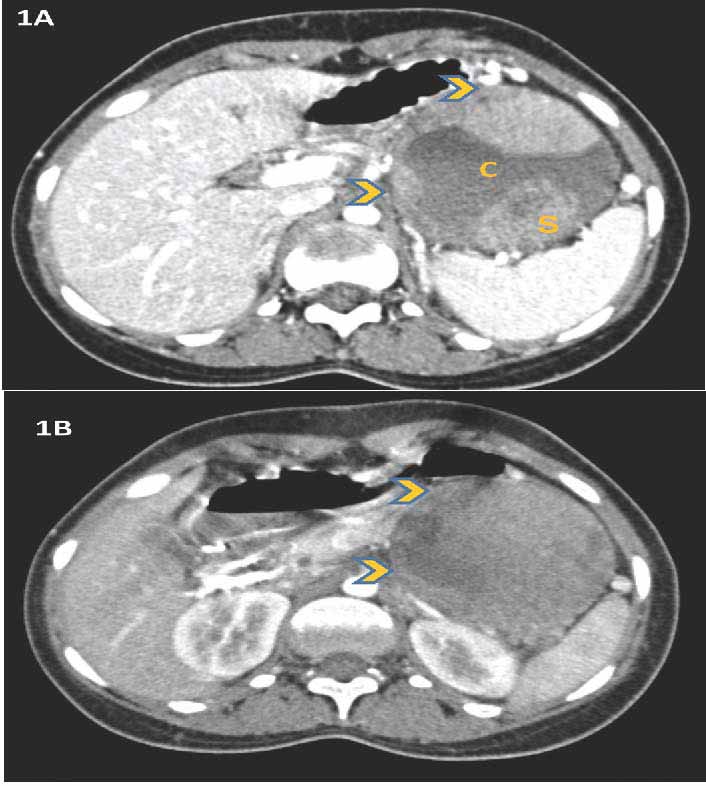
Figure 1: CT scan abdomen and pelvis shows an enhancing, rounded, well defined, cystic (C) and solid (S) tumor located in proximity to the spleen; (1A) and supero-anterior to the left kidney; (1B) (arrowheads).
Per-operatively, there was a large cyst at the tail of the pancreas adherent to the splenic artery and vein. The peritoneal cavity, liver and spleen were normal. The resected mass, sent for frozen section, was globular, 10 cm in diameter, well circumscribed, and solid brown on cut surface with a white nodule (Fig. 2A). There was no definite infiltration into the surrounding tissues. Intra-operatively, imprint cytology, showed a cellular tumor with sheets of monomorphic cells loosely aggregated around arborising vessels (Fig. 2B). The cells had uniform oval nuclei with occasional grooves and a pale cytoplasm. A diagnosis of solid pseudopapillary neoplasm of the pancreas was made. The patient underwent a spleen-preserving distal pancreatectomy of the lesion.
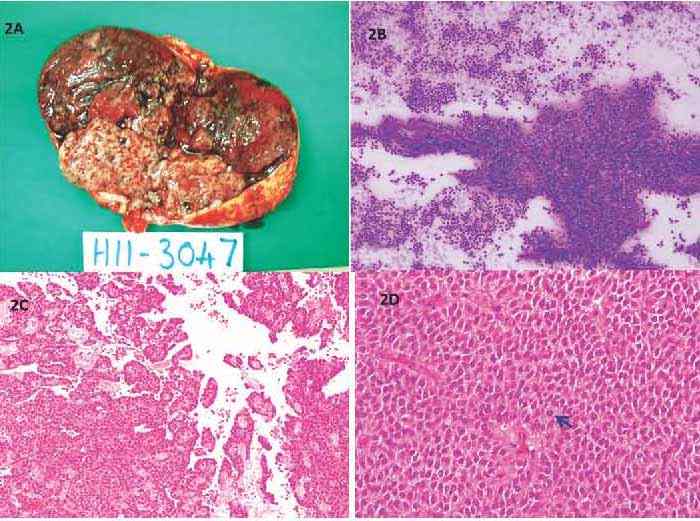
Figure 2: Solid pseudopapillary neoplasm (SPN) of the pancreas; macroscopic and microscopic appearance. (A) globular encapsulated tumor with solid brown areas and a whitish nodule; (B) Intraoperative imprint cytology shows the pseudopapillary arrangement of uniform cells around arborising vessels (Diff Quik × 40); (C) The tumor forms pseudorosettes and pseudopapillae projecting into cystic spaces (H&E × 40); (D)Tumor cells are polygonal with eosinophilic cytoplasm, vesicular nuclei with grooves with minimal atypia and occasional mitosis (arrow) (H&E × 400).
Microscopically, there were solid and cystic areas with pseudorosetting and pseudopapillae formed by polygonal cells with eosinophilic cytoplasm, vesicular nuclei with grooves as well as minimal atypia and occasional mitosis (Figs. 2C-D). There were no necrobiotic cell nests. Perineural and capsular invasion were present but there was no vascular invasion. Immunophenotypic expression of beta catenin in the nuclei and absence of e-cadherin staining were the hallmarks (Figs. 3A-B). In addition, the tumor expressed progesterone receptor (Fig. 3C), vimentin, CD10 and CD56. Focal synaptophysin and chromogranin positivity was seen (Fig. 3D). Alpha-1 antitrypsin was focally expressed, and Ki67 proliferation rate was <1%. A diagnosis of solid pseudopapillary neoplasm of the pancreas was made. Features of aggressive behavior included size >5 cm, mitosis, capsular and perineural invasion.
The patient made an uneventful post-operative recovery. Since the disease was localized and the excision was complete, the patient was not given adjuvant treatment, and shall be on regular follow-up.
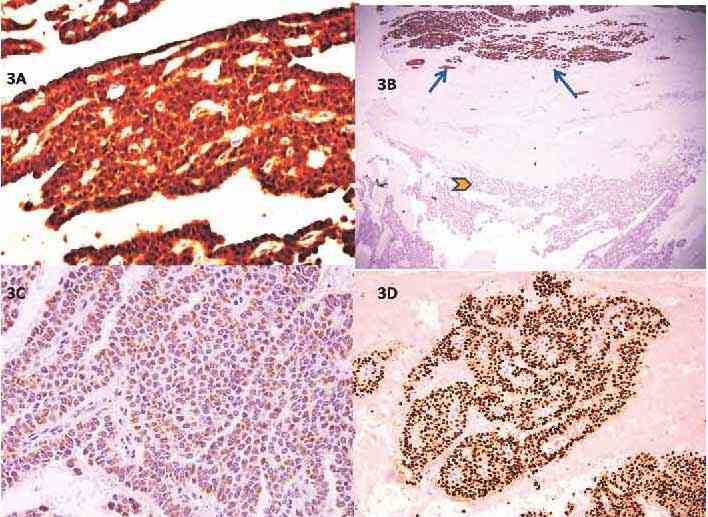
Figure 3: Immunophenotypic features of SPN. (A) Strong nuclear positivity for ß-catenin (DAB × 400); (B) The surrounding pancreatic tissue expressing e-cadherin (arrows) while the tumor is negative (arrowhead), (DAB × 40); (C) Weak, focal, granular cytoplasmic positivity for chromogranin in the tumor cells (DAB × 400); (D) Diffuse nuclear progesterone receptor (PR) positivity in the tumor cells (IHC with DAB ×100).
Discussion
Incidental pancreatic cystic lesions have an incidence of 0.2% to 0.7%.1 Solid pseudopapillary neoplasm (SPN) or Frantz tumor of the pancreas is a rare pancreatic tumor of uncertain lineage seen in young, predominantly Asian females, first reported by Frantz in 1959.2 Upper abdominal pain is the dominant symptom, occasionally associated with a mass, nausea, vomiting, or other gastrointestinal symptoms.3 The CT scan reveals a well circumscribed, vascularized mass with solid and cystic areas sometimes with calcification, located more commonly in the tail of the pancreas.3 On endoscopic ultrasound, it is often an echo-poor mass sometimes with mixed echogenicity. The differential diagnoses include pseudocysts and other cystic neoplasms. Cytologic features on fine needle aspiration of SPN have been described in the literature,4 and for head of pancreas lesions, endoscopic-guided FNAC is useful.6
In our case, intraoperative cytologic diagnosis proved invaluable for determining the extent of surgery. The tissue sections show a tumor made up of epithelial cells with minimal atypia forming pseudorosettes and pseudopapillae with areas of cystic breakdown. Calcification may be present. SPN shows strong nuclear and cytoplasmic beta catenin immunoreactivity in 100% cases and loss of membrane staining for E-cadherin (due to activation of the Wnt signaling pathway).5 This is the most definitive way of differentiation from pancreatic endocrine neoplasms (PEN) as there may be considerable overlap in other markers like α1-antitrypsin, α1-antichymotrypsin, NSE, synaptophysin, progesterone receptor (PR), carcinoembryonic antigen, pan CK, vimentin, CD10, CD56, and cyclin D1.7
In FNAC, a combination of ß-catenin (nuclear positivity), e-cadherin (membrane negativity) and CD 10 positivity is most discriminatory, favoring diagnosis of SPN over pancreatic endocrine neoplasm.8 Focal chromogranin positivity in a few SPN does not detract from the diagnosis because in PEN it is diffusely positive.8 Other differentials include pancreatoblastoma and acinar cell carcinoma which can be distinguished by their clinical and morpho-immunophenotypic characteristics.2,9 When SPN undergoes extensive cystic change, sampling from the preserved solid areas is crucial in order to distinguish it from a pseudocyst. Metastatic renal cell carcinoma and malignant melanoma may be microscopic look-alikes but have entirely different immunoexpressions. While the expression of a hormone receptor (PR) is not specific for diagnosis of SPN, it may offer an explanation for the occasional anecdotal report of tumor rupture during pregnancy.10
Histopathologic criteria of malignancy are not well established but size >5 cm, nuclear atypia, vascular, perineural and invasion of surrounding structures and high proliferation rate may predict aggressive behavior.11 Controversy exists about the cell of origin with proposed origins from acinar, ductal, endocrine and multipotential primordial cells.12 The cells do not resemble cells of the embryonic or adult pancreas on morphology. Ultrastructure and immunohistochemistry have added little to clarify this enigma.9 Mutations in the ß-catenin gene (CTNNB1) exon 3 are responsible for the immunohistochemical demonstration of nuclear positivity and the loss of e-cadherin expression due to aberrant activation of the Wnt-B- catenin pathway.13 SPN-like lesions have been linked to ß-catenin mutations in a mice model and it is suggested that the cell of origin may lie in the ductal compartment.14 Recently, a centroacinar cell origin has been proposed because of uniform expression of DOG-1.15
Good surgical resection is the mainstay of treatment and has included enucleation, Whipple’s resection or subtotal pancreatectomy depending upon the size and extent of the tumor.11,16 They run an indolent clinical course; recurrences are uncommon but up to 15% can show aggressive behavior.17 In a study of 24 cases, including two patients presenting with advanced disease and R1 resection with only one mortality, the authors recommend as complete resection as possible with follow-up second stage surgeries.17 The overall five-year survival rate is >95%.18 This merits spleen-saving surgery as was performed in the case being reported. Older male patients, atypical histopathology (large tumors, diffuse growth, cellular/nuclear atypia, mitotic activity, necrosis, invasion/metastasis) and incomplete resection may have a higher risk of recurrence and death, deserving particular attention.19 Chemo-radiotherapy with cisplatin, 5-fluorouracil and gemcitabine has been tried in the rare cases with metastases.20 Even with advanced disease, aggressive initial therapy gives excellent long term survival.21 The rare occurrence of peritoneal carcinomatosis may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC).22 Radiofrequency ablation (RFA) offers a viable option for safe and effective treatment for unresectable liver metastasis.23
Conclusion
This case report on pseudopapillary neoplasm of the pancreas highlights the enigmatic histogenesis of this indolent neoplasm and the need to distinguish it from more aggressive pancreatic tumors that may be considered in the morphologic differential diagnosis. Characteristic mutations in the ß-catenin gene have proven useful in providing specificity of diagnosis. The excellent long term prognosis of this potentially malignant tumor underscores the need for an accurate morpho-immunophenotypic diagnosis (pre- or intra-operative) for planning definitive surgery that is curative in a majority of patients.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Edirimanne S, Connor SJ. Incidental pancreatic cystic lesions. World J Surg 2008 Sep;32(9):2028-2037.
2. Bosman F, Carneiro F, Hruban R, Theise N, eds. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010
3. Nguyen NQ, Johns AL, Gill AJ, Ring N, Chang DK, Clarkson A, et al. Clinical and immunohistochemical features of 34 solid pseudopapillary tumors of the pancreas. J Gastroenterol Hepatol 2011 Feb;26(2):267-274.
4. Raddaoui E. Clinical utility and diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions: Saudi Arabian experience. Acta Cytol 2011;55(1):26-29.
5. Serra S, Chetty R. Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol 2008 Nov;61(11):1153-1159.
6. Song JS, Yoo CW, Kwon Y, Hong EK. Endoscopic ultrasound-guided fine needle aspiration cytology diagnosis of solid pseudopapillary neoplasm: three case reports with review of literature. Korean J Pathol 2012 Aug;46(4):399-406.
7. Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol 2008 Feb;39(2):251-258.
8. Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: a limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol 2009 Dec;132(6):831-839.
9. Chakhachiro ZI, Zaatari G. Solid-pseudopapillary neoplasm: a pancreatic enigma. Arch Pathol Lab Med 2009 Dec;133(12):1989-1993.
10. Huang SC, Wu TH, Chen CC, Chen TC. Spontaneous rupture of solid pseudopapillary neoplasm of the pancreas during pregnancy. Obstet Gynecol 2013 Feb;121(2 Pt 2)(Suppl 1):486-488.
11. Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol 2010 Mar;16(10):1209-1214.
12. Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, Rahier J, et al. Solid and pseudopapillary tumor of the pancreas–review and new insights into pathogenesis. Am J Surg Pathol 2006 Oct;30(10):1243-1249.
13. Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol 2010 Feb;16(8):1025-1030.
14. Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology 2008 Oct;135(4):1288-1300.
15. Bergmann F, Andrulis M, Hartwig W, Penzel R, Gaida MM, Herpel E, et al. Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms–novel evidence for a histogenetic relationship. Hum Pathol 2011 Jun;42(6):817-823.
16. de Castro SM, Singhal D, Aronson DC, Busch OR, van Gulik TM, Obertop H, et al. Management of solid-pseudopapillary neoplasms of the pancreas: a comparison with standard pancreatic neoplasms. World J Surg 2007 May;31(5):1130-1135.
17. Guo N, Zhou QB, Chen RF, Zou SQ, Li ZH, Lin Q, et al. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg 2011 Dec;54(6):368-374.
18. Igbinosa O. Pseudopapillary tumor of the pancreas. An algorithmic approach. JOP 2011 May;12(3):262-265.
19. Ansari D, Elebro J, Tingstedt B, Ygland E, Fabricius M, Andersson B, et al. Single-institution experience with solid pseudopapillary neoplasm of the pancreas. Scand J Gastroenterol 2011 Dec;46(12):1492-1497.
20. Sperti C, Berselli M, Pasquali C, Pastorelli D, Pedrazzoli S. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J Gastroenterol 2008 Feb;14(6):960-965.
21. Jo H, Park WK, Lee NH, Choi JH. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J Pediatr Hematol Oncol 2000;29:851-853.
22. Honore C, Goere D, Dartigues P, Burtin P, Dumont F, Elias D. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz’s tumour) of the pancreas treated with HIPEC. Anticancer Res 2012 Mar;32(3):1069-1073.
23. Li JX, Wu H, Huang JW, Prasoon P, Zeng Y. Synchronous intraoperative radiofrequency ablation for multiple liver metastasis and resection of giant solid pseudopapillary tumors of the pancreas. Chin Med J (Engl) 2012 May;125(9):1661-1663.
|
|