Prolonged or extensive use of glucocorticoids can cause Cushing’s syndrome, striae, hypertension, hypothalamic-pituitary-adrenal (HPA) axis suppression, and other systemic adverse effects. Although most common with oral or parenteral route, uncontrolled topical therapy with moderate- to high-potency glucocorticoids can cause steroid-associated adverse effects and the possibility of steroid withdrawal symptoms.1,2 However, iatrogenic Cushing’s syndrome and secondary adrenal failure as a result of topical steroids is unusual.3 Pediatric patients may be more susceptible to systemic toxicity from equivalent doses of topical corticosteroid due to their larger skin surface to body mass ratios.4,5 This is more likely when higher potency topical corticosteroids are used over extensive areas.4
Clobetasol propionate cream is an analog of prednisolone with high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity that is widely used to treat dermatologic diseases. Skin on the face, axilla, and groin appears to be most susceptible to the adverse long-term effects of topical clobetasol.6
At least 43 cases of iatrogenic Cushing’s syndrome in children and adults receiving topical clobetasol have been described in the last 35 years.1 Ozon et al,2 presented three infants with symptoms of Cushing’s syndrome during glucocorticoid therapy for diaper dermatitis, one infant also developed steatohepatitis, an uncommon side effect of topical corticosteroids.
In this report we describe significant growth impairment on both weight and height with HPA axis suppression in a 17-month-old boy caused by excessive administration of clobetasol to treat diaper dermatitis.
Case Report
A 17-month-old boy with unremarkable birth history was referred to outpatients pediatric endocrine clinic for management of growth retardation. He was otherwise well with no significant past medical or family history. At birth, his weight and height were 2.7kg (5th percentile), 49cm (5th percentile), respectively. His growth was regular until he was 11-months-old. On presentation, his weight was 9.7kg (5th percentile) and height was 72cm (-3.6 SD below mean for age and sex). His head circumference was 48cm (50th–75th percentile). He had a heart rate of 110 beats/min and blood pressure of 105/60mmHg (>95 percentile by age, sex and length). The growth chart showed growth retardation that began at 11 months. He had a grossly moon-like face [Figure 1], striae and hypertrichosis [Figure 2] with thin skin in his genital area. There was no truncal obesity. Laboratory analysis including, complete blood cell count, liver function tests, chemistry panel, and thyroid function tests revealed no abnormalities. Baseline morning cortisol level was less than 0.3ng/ml (normal range, 60 to 280ng/ml). The peak cortisol response to the standard-dose adrenocorticotropic hormone (ACTH) test (tetracosactrin: 250µg)7,8 was 0.4ng/ml (N>180ng/ml).9 Adrenal insufficiency was considered because of the poor response to the ACTH stimulation test.
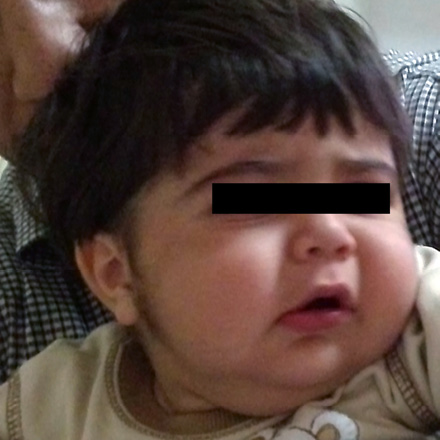
Figure 1: Cushingoid features including moon face can be seen in the patient due to overexposure to topical clobetasol.
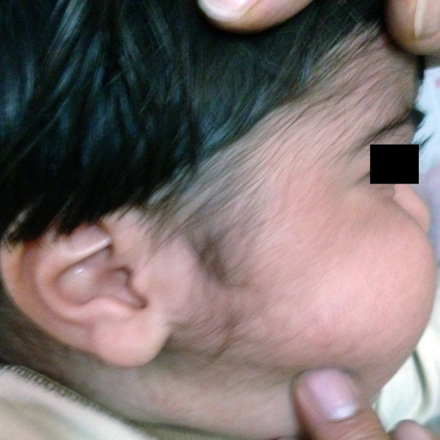
Figure 2: Hypertrichosis can be seen.
On further enquiry, his mother admitted to using continued clobetasol propionate ointment, approximately 2g twice-daily during the seven months prior to admission, after the drug was prescribed for treatment of diaper dermatitis. Thereafter, he had continued receiving unprescribed topical clobetasol for an extended period. According to physical signs, including a moon-like face, hyperthricosis, and thin skin in the genital area, and laboratory tests a diagnosis of iatrogenic Cushing’s syndrome followed by secondary adrenal failure was considered. Appropriate replacement doses of hydrocortisone (10mg/m2/24hr)9,10 twice-daily (2mg in the morning and 1mg at 3pm) was given to prevent an adrenal crisis and the dose was tapered over two months. The infant was evaluated closely.
Plasma cortisol concentrations returned to normal range (101.2ng/ml) within six weeks of stopping the oral physiologic dose of hydrocortisone. Clinical symptoms resolved after a few months including a significant decrease in facial puffiness and blood pressure 70/40mmHg (<50 percentile by age ,sex and height) and an increase in height occurred. At 22-months-old, his weight was 10kg (5th percentile) and height was 79cm (5th percentile).
Discussion
Topical corticosteroids which are widely used to treat diaper dermatitis may lead to the development of severe side effects. In this case, an infant overexposed to topical corticosteroid developed growth impairment and surprisingly poor weight gain within a few months. The patient had severe adrenal suppression, which developed after daily use of 4g clobetasol propionate (28g/week) ointment, which was high for an 11-month-old infant. This data is consistent with a number of previous reports, which have shown that 2g per day application of clobetasol propionate 0.05% ointment can cause a decrease in morning cortisol after only a few days.7,11 Ohman et al,7 have demonstrated that use of 7.5g per week of steroids can lead to Cushing’s syndrome and HPA axis suppression. In our case, the medical history and clinical evaluation did not indicate the etiology of poor weight gain. Height arrest correlated well to the increased topical steroids exposure, but it was unclear why this patient developed poor weight gain rather than excessive weight gain.
Numerous similar cases of Cushing’s syndrome following oral, topical, inhaled, or intranasal administration of steroids were found in the literature. However, most of these cases presented with progressive weight gain and linear growth retardation.11-16 For example, Dutta et al,9 presented iatrogenic Cushing’s syndrome in a six-year-old girl whose weight, height and body mass index were in the 3rd percentile, 50–75th percentile, and 85–97th percentile, respectively. Semiz et al,13 previously reported two infants with iatrogenic Cushing’s syndrome due to abuse of the clobetasol propionate. Both infants presented with rapid weight gain and obesity. Messina et al,16 reported progressive weight gain (10kg) over six months in a 7.6-year-old boy with Cushing’s syndrome due to dexamethasone 2% ocular drops. Findlay et al,17 described two cases who developed iatrogenic Cushing’s syndrome induced by prolonged use of intranasal betamethasone. The first case was a seven-year-old boy whose weight during the two years prior to admission had increased from the 50th to the 97th centile with height falling from the 10th to the 3rd centile. The second case had Down’s syndrome and over one year his weight increased from the 3rd to above the 95th centile with height falling from the 10th to the 3rd centile. Baş et al,18 described a four-month-old male who presented with rapid weight gain and growth retardation following steroid nasal drops. Very few case reports documenting poor weight gain in iatrogenic Cushing’s syndrome are available in the paediatric literature.6,19 Coureau et al,6 reported an infant with atopic dermatitis, food allergies and failure to thrive treated with topical corticosteroids. The authors concluded that growth retardation was due to food allergies, atopic dermatitis, and suppression of the HPA axis.
In our case, the diagnostic investigations did not indicate the etiology of poor weight gain. We agree with Coureau et al,6 that HPA axis suppression may be an independent factor negatively influencing weight growth in infants.
The purpose of reporting this case was to highlight that topical steroids may result in iatrogenic Cushing’s syndrome without excessive weight gain.
Conclusion
Cushing’s syndrome following topical steroids is well known. However, poor weight gain as a result of topical steroids is extremely rare. Early diagnosis requires a high degree of suspicion. When assessing a child with growth failure it is important to take a careful drug history and ask specifically about the use of any steroid-containing drugs. Parents also need to be made aware of the potential side effects of prolonged use of topical glucocorticoidsis in infants.
Disclosure
The authors declared no conflict of interest. No funding was received for this work.
Acknowledgements
The authors would like to thank the parents of the patient for their cooperation.
references
- Tempark T, Phatarakijnirund V, Chatproedprai S, Watcharasindhu S, Supornsilchai V, Wananukul S. Exogenous Cushing’s syndrome due to topical corticosteroid application: case report and review literature. Endocrine 2010 Dec;38(3):328-334.
- Ozon A, Cetinkaya S, Alikasifoglu A, Gonc EN, Sen Y, Kandemir N. Inappropriate use of potent topical glucocorticoids in infants. J Pediatr Endocrinol Metab 2007 Feb;20(2):219-225.
- Siklar Z, Bostanci I, Atli O, Dallar Y. An infantile Cushing syndrome due to misuse of topical steroid. Pediatr Dermatol 2004 Sep-Oct;21(5):561-563.
- Munro DD; MunroDD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica 1976;152(152)(Suppl 1):173-180.
- Güven A, Gülümser O, Ozgen T. Cushing’s syndrome and adrenocortical insufficiency caused by topical steroids: misuse or abuse? J Pediatr Endocrinol Metab 2007 Nov;20(11):1173-1182.
- Coureau B, Bussières JF, Tremblay S. Cushing’s syndrome induced by misuse of moderate- to high-potency topical corticosteroids. Ann Pharmacother 2008 Dec;42(12):1903-1907.
- Ohman EM, Rogers S, Meenan FO, McKenna TJ. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med 1987 Jul;80(7):422-424.
- Mahlab-Guri K, Asher I, Gradstein S, Zung A, Radian-Sade S, Elbirt D, et al. Inhaled fluticasone causes iatrogenic cushing’s syndrome in patients treated with Ritonavir. J Asthma 2008 Oct:48(8):860-863.
- Dutta D, Shivaprasad KS, Ghosh S, Mukhopadhyay S, Chowdhury S. Iatrogenic Cushing’s syndrome following short-term intranasal steroid use. J Clin Res Pediatr Endocrinol 2012 Sep;4(3):157-159.
- DeVile CJ, Stanhope R. Hydrocortisone replacement therapy in children and adolescents with hypopituitarism. Clin Endocrinol (Oxf) 1997 Jul;47(1):37-41.
- Gilbertson EO, Spellman MC, Piacquadio DJ, Mulford MI. Super potent topical corticosteroid use associated with adrenal suppression: clinical considerations. J Am Acad Dermatol 1998 Feb;38(2 Pt 2):318-321.
- Perry RJ, Findlay CA, Donaldson MD. Cushing’s syndrome, growth impairment, and occult adrenal suppression associated with intranasal steroids. Arch Dis Child 2002 Jul;87(1):45-48.
- Semiz S, Balci YI, Ergin S, Candemir M, Polat A. Two cases of Cushing’s syndrome due to overuse of topical steroid in the diaper area. Pediatr Dermatol 2008 Sep-Oct;25(5):544-547.
- Kedem E, Shahar E, Hassoun G, Pollack S. Iatrogenic Cushing’s syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma 2010 Sep;47(7):830-831.
- Fuchs M, Wetzig H, Kertscher F, Täschner R, Keller E. Iatrogenic Cushing syndrome and mutatio tarda caused by dexamethasone containing nose drops. 1999 Jul;47(7):647-50. (German)
- Messina MF, Valenzise M, Aversa S, Arrigo T, De Luca F Iatrogenic Cushing syndrome caused by ocular glucocorticoids in a child. BMJ Case Rep. 2009;2009. pii: bcr11.2008.1224
- Findlay CA, Macdonald JF, Wallace AM, et al. Childhood Cushing’s syndrome induced by betamethasone nose drops, and repeat prescriptions. BMJ1998;317:739–40.
- Baş VN, Cetinkaya S, Aycan Z. Iatrogenic Cushing syndrome due to nasal steroid drops. Eur J Pediatr 2012 Apr;171(4):735-736.
- Patel L, Wales JK, Kibirige MS, Massarano AA, Couriel JM, Clayton PE. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Arch Dis Child 2001 Oct;85(4):330-334.