|
Abstract
We report two cases that presented with unexplained acute renal failure and hyperuricemia and were subsequently diagnosed with T-cell acute lymphoblastic leukemia. The patients improved with conservative therapy without the need for dialysis. Case 1 is the youngest case of acute lymphoblastic leukemia with spontaneous tumor lysis syndrome reported to date, and Case 2 presented with spontaneous tumor lysis syndrome without hyperleukocytosis.
Keywords: Acute leukemia; Hyperuricemia; Dialysis; Child; Tumor lysis.
Intruduction
Tumor lysis syndrome (TLS) is a well-described potentially lethal condition that results from rapid destruction of neoplastic cells with release of cellular breakdown products into the systemic circulation.1 It commonly occurs following therapy of malignancies such as acute leukemias and lymphomas.1,2 However, acute spontaneous TLS is a rare phenomenon, and it is very rare in T cell acute lymphoblastic leukemia (ALL). We report two cases that presented with unexplained acute renal failure (ARF) and hyperuricemia and were subsequently diagnosed with T-cell (ALL).
Case 1
A 4-year-old boy presented with a 15-day history of fever, progressively increasing neck swelling and oliguria. On examination, there was mild pallor along with cervical and axillary lymphadenopathy, and hepatosplenomegaly (liver 8 cm below costal margin with span of 12 cm, spleen 9 cm below costal margin). The child was investigated. Hemogram showed hemoglobin of 10.8 g/dL, total leukocyte count (TLC) of 182,000/cu mm, and platelet count of 83,000/cu mm. Renal function tests along with biochemical parameters are shown in figure 1. Chest radiograph (CXR) showed mediastinal widening, along with left-sided pleural effusion (Fig. 2). Ultrasonography demonstrated hepatosplenomegaly, mild bilateral hydronephrosis, and collapse of the underlying left lung with left-sided pleural effusion. Echocardiography showed minimal pericardial effusion.
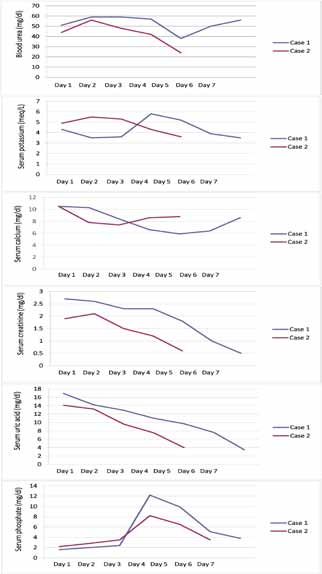
Figure 1: Serial biochemical parameters of both the cases.
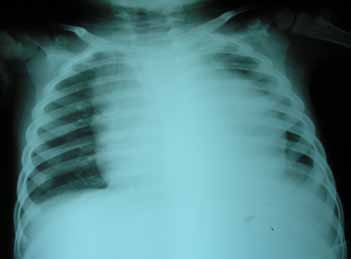
Figure 2: Chest radiograph showing mediastinal widening and left sided pleural effusion.
The child was started on intravenous fluid (3 litre/m2/day) with urinary alkalinization and allopurinol, considering the possibility of spontaneous TLS based on initial laboratory parameters (Fig. 1). Furosemide and mannitol were given when required. Oliguria (urine output: 0.5 - 1 mL/kg/hr) was relative and intermittent during the initial 72 hrs of admission. There was mild facial puffiness but no other signs of fluid overload. Hemodialysis was planned in case the child would develop electrolyte abnormality, fluid overload or uremia. Gradually, the urine output improved and the uric acid level began to decrease. At this point, the child developed other biochemical features of TLS such as hyperkalemia and hyperphosphatemia. The fluid therapy was further escaled to 3.5 litre/m2/day and the child was observed for features of fluid overload. Gradually, the TLS settled. Peripheral blood smear and bone marrow at this time revealed 78% blasts. Bone marrow aspirate showed near total replacement by blasts. On immunophenotyping, blasts were negative for CD34, cMPO, cCD79a, and positive for CD45, cCD3, CD5, CD8, CD2, and CD7, consistent with T cell ALL. The child was started on chemotherapy after day 7 of hospitalization and is currently in remission.
Case 2
A 12-year-old male child presented with one-month history of fever, cough, abdominal pain, and three-day history of oliguria. On examination, there was mild pallor along with cervical and axillary lymphadenopathy, and hepatomegaly (liver 4 cm below costal margin with span of 10 cm). Six months back, he was treated with anti-tubercular drugs as CXR was showing mediastinal widening and computed tomography scan was showing mediastinal lymphadenopathy. Hemogram showed hemoglobin of 10 g/dL, total leukocyte count (TLC) of 96,400/cu mm, and platelet count of 16,000/cu mm. Renal function tests along with biochemical parameters are shown in Fig. 1. Repeat CXR was showing mediastinal widening as above. Ultrasonography demonstrated hepatomegaly with altered echotexture, abdominal lymphadenopathy, and normal kidney size with poor corticomedullary differentiation. Keeping the possibility of spontaneous TLS, the child was managed similarly as the previous case. Peripheral blood smear revealed 30% blasts of lymphoid morphology. Bone marrow showed infiltration by 90% blasts. On immunophenotyping, blasts were negative for CD34, cMPO, cCD79a and positive for CD45, cCD3, CD5, CD7, CD8 and CD1a, consistent with T cell ALL. He received chemotherapy, and is currently in remission.
Discussion
Acute TLS is an oncologic emergency that can cause electrolyte disturbances, metabolic acidosis, ARF and in fulminant cases, seizure, arrhythmia, and death. It is usually observed after initiation of cytotoxic chemotherapy, and the risk factors for its development include tumor type, tumor burden, renal function, baseline uric acid level, and rapid cytoreduction therapy.3 The initiation of chemotherapy results in rapid release of intracellular ions (phosphorus and potassium), metabolic products of proteins, and nucleic acids into the bloodstream.4 Hyperuricemia results from the catabolism of nucleic acids by the action of xanthine oxidase.5 Hypocalcemia occurs as a result of hyperphosphatemia and renal deposition of calcium phosphate crystals.6 Deposition of uric acid, xanthine, and calcium phosphate crystals in the renal tubules can result in ARF, especially when there is reduced renal perfusion or an acidic urine pH. The important distinction between spontaneous TLS and post-chemotherapy TLS is that, the former is not associated with hyperphosphatemia but with hyperuricemia leading to ARF. One suggestion for this results from the fact that high cell turnover rate leads to high uric acid levels through nucleobase turnover, but the tumor reuses the released phosphate for growth of new tumor cells. In post-chemotherapy TLS, tumor cells are destroyed and no new tumor cells are synthesized.
Case 1 is the youngest case of spontaneous TLS reported for ALL. Sharma et al. described a 20-year-old patient with ALL and spontaneous TLS, managed with intravenous fluids, platelet concentrates, allopurinol and heparin free hemodialysis on alternate days till renal functions recovered.7 Akoz et al. reported a 17-year-old patient with T-cell ALL having spontaneous TLS and pericardial involvement. She was in non-oliguric ARF with extreme hyperuricemia, but responded to intravenous hydration and allopurinol.8 Chen and Chuang, reported a 14-year-old male with precursor B-cell ALL and spontaneous TLS, managed supportively with allopurinol and rasburicase as the patient presented with transient non-oliguric ARF. After supportive therapy, the ALL went into remission spontaneously. One month later, his ALL recurred, without tumor lysis syndrome, and he achieved a second remission with conventional chemotherapy.9
Careful monitoring of fluid intake and output, electrolyte management and management of hypertension are the cornerstone of management of renal dysfunction associated with acute TLS. Secondary management of hyperuricemia, hyperphosphatemia and avoidance of uric acid/xanthine renal crystallization and calcium phosphate renal deposition are also critically important in the prevention of further renal damage.10 The initial presentations of hyperphosphatemia, hyperkalemia, metabolic acidosis, hyperuricemia, and ARF and the subsequent discovery of T cell ALL in our patient are consistent with spontaneous TLS. The risk factors for spontaneous TLS in the presented cases were: hyperleukocytosis (surprisingly not in Case 2), and large tumor burden (both cases). Both of them developed hyperuricemia, though no other biochemical features of TLS were evident at admission, but developed later. Spontaneous TLS may occur without hyperleukocytosis. It is reversible when recognized early and treated aggressively, and dialysis may not be needed in all the cases.
Conclusion
Spontaneous tumor lysis syndrome may occur without hyperleukocytosis in T cell ALL. It is reversible when recognized early and treated aggressively, and dialysis may not be needed in all the cases.
Acknowledgements
The authors reported no conflict of interest and no funding was received in this work.
References
1. Agnani S, Gupta R, Atray NK, Vachharajani TJ. Marked hyperuricemia with acute renal failure: need to consider occult malignancy and spontaneous tumour lysis syndrome. Int J Clin Pract 2006 Mar;60(3):364-366.
2. Lotfi M, Brandwein JM. Spontaneous acute tumor lysis syndrome in acute myeloid leukemia? A single case report with discussion of the literature. Leuk Lymphoma 1998 May;29(5-6):625-628.
3. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol 2008 Jun;26(16):2767-2778.
4. Tannock I. Cell kinetics and chemotherapy: a critical review. Cancer Treat Rep 1978 Aug;62(8):1117-1133.
5. Van den Berghe G. Purine and pyrimidine metabolism between millennia: what has been accomplished, what has to be done? Adv Exp Med Biol 2000;486:1-4.
6. Wechsler DS, Kastan MB, Fivush BA. Resolution of nephrocalcinosis associated with tumor lysis syndrome. Pediatr Hematol Oncol 1994 Jan-Feb;11(1):115-118.
7. Sharma SK, Malhotra P, Kumar M, Sharma A, Varma N, Singh S. Spontaneous tumor lysis syndrome in acute lymphoblastic leukemia. J Assoc Physicians India 2005 Sep;53:828-830.
8. Akoz AG, Yildirim N, Engin H, Dagdas S, Ozet G, Tekin IO, et al. An unusual case of spontaneous acute tumor lysis syndrome associated with acute lymphoblastic leukemia: a case report and review of the literature. Acta Oncol 2007;46(8):1190-1192.
9. Chen RL, Chuang SS. Transient spontaneous remission after tumor lysis syndrome triggered by a severe pulmonary infection in an adolescent boy with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2009 Jan;31(1):76-79.
10. Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004 Oct;127(1):3-11.
|
|