| |
Abstract
Objectives: Toxoplasma gondii is a worldwide prevalent zoonotic parasite which causes toxoplasmosis. An appropriate vaccine for animals could interrupt the circle between animals and humans. Our previous study showed that excreted/secreted antigens (E/SA), derived from the peritoneum of mice infected with T. gondii tachyzoites could be considered as a good candidate for animal vaccination. Interleukin-10 (IL-10) inhibits proliferation of B and T lymphocytes and induces homeostasis in immune system responses. However, since IL-10 has also been shown to suppress the killing of T. gondii by human macrophages, the aim of this study was to evaluate IL-10 serum levels after vaccination with T. gondii E/SA prepared in vivo.
Methods: T. gondii tachyzoites were inoculated in the peritoneum of mice and harvested E/SA were used as a vaccine, with and without adjuvant, in T. gondii infected and un-infected mice. IL-10 serum levels were evaluated using the ELISA technique.
Results: The data showed that although serum levels of IL-10 were not changed at the early phases, they were elevated at the end phases of vaccination with T. gondii E/SA.
Conclusion: Based on these and our previous results, it can be concluded that in vivo prepared T. gondii E/SA could be considered as a good candidate for animal vaccination.
Keywords: Toxoplasma gondii; Vaccination; IL-10; Excreted/secreted antigens.
Introduction
Toxoplasma gondii (T. gondii) is the main cause of the toxoplasmosis.1,2 This parasite rarely causes any clinical presentations in infected immuno-competent individuals; however, immuno-suppressed adults or congenitally infected infants may suffer from the severe pathological effects of the disease.3 T. gondii can be transmitted by eating or drinking infected meat or milk, respectively, during contact with food, water or dust contaminated with cat feces, or by handling infected animals.4,5 Therefore, the immunization of the animals against T. gondii could be effective in reducing the risk of human contamination.
Purified and recombinant proteins, and DNA of T. gondii,6-8 as well as whole inactive tachyzoites,9 have been used to make an appropriate vaccine in animals. However, none of the vaccines have induced successful immunization against the parasite. Excreted/secreted antigens of Toxoplasma gondii tachyzoites (E/SA), which represent more than 90% of the parasite circulated antigens,2,10 might be the first target of the host immune system. Namely, it seems that E/SAs play a crucial role in inducing appropriate humoral and cellular immune responses against T. gondii.10
Some vaccination studies which used E/SA derived from in vitro T. gondii culture,9,11 reported that although it might be considered as a good candidate for immunization against T. gondii, it cannot achieve 100% protection.9,11 This perhaps may be due to a lack of appropriate glycosylation of in vitro prepared antigens, which has been observed (in vivo) in Toxoplasma gondii.12 Hence, in vivo prepared T. gondii E/SA may represent a better choice for a vaccine. Our previous study already showed that T. gondii E/SA derived from the peritoneum of mice can be considered as a good candidate for animal vaccination.2
Interleukin-10 (IL-10) plays a key role in the regulation of many functions of the immune system.13 This cytokine inhibits the proliferation of B and T lymphocytes and induces homeostasis in immune system responses.13 Additionally, IL-10 suppresses the killing of T. gondii by human macrophages.14 Therefore, it is important that a vaccine against Toxoplasma gondii does not elevate IL-10 production, at least not during the first few days after vaccination. Hence, the aim of this study was to evaluate IL-10 serum levels in mice after vaccination with the in vivo prepared T. gondii E/SA.
Methods
The study was carried out on 8-10-week-old Balb/C female inbred mice obtained from an animal house at the Rafsanjan University of Medical Sciences and maintained under standard conditions. Each investigation group consisted of 8-10 mice and received different injections. The designed groups were: A: No injection; B: 10 μg prepared E/SA; C: 10 μg prepared E/SA and 100 μl completed Freunds’ adjuvant; D: 100 μl completed Freunds’ adjuvant; E: (T. gondii infected) 10 μg prepared E/SA; F: (T. gondii infected) 10 μg prepared E/SA and 100 μl completed Freunds’ adjuvant; G (T. gondii infected): 100 μl completed Freunds’ adjuvant; and H: 100 μl normal salin solution. Blood samples were collected from the tail vein 3, 7, 14, 28 and 56 days after immunization of the mice and sera were stored at -20ºC until IL-10 analysis. Pre-immune serum samples (group A) were used as negative controls.
Antigen (E/SA) preparation was done 3 days after the infection of mice by peritoneal inoculation with T. gondii tachyzoites (RH strain), peritoneal fluids were taken, centrifuged (1000×g for 15 min) and E/SA supernatants were filtered and saved at -20ºC for future use. The cytokine assay was done using IL-10 serum levels which were detected using ELISA technique (eBioscience, ESP), immediately after blood collection. Assays were performed according to the manufacturer’s guidelines. The sensitivity of the kit was 2 pg/ml and inter- and intra-assay assessments of reliability of the kit were conducted.
Statistical analyses were done to determine the differences between different groups with the same time interval after the immunization of mice and between same groups with different time intervals after the immunization of mice, the data was analyzed using ANOVA and repeated measures ANOVA tests, respectively. A p value less than 0.05 was considered significant.
Results
The (T. gondii infected) mice in groups E, F and G died after 5 days, while uninfected mice lived, as expected. Cytokine assays revealed that the serum levels of IL-10 did not change in groups A and H during different times intervals after the immunization of mice; while they were increased in groups E, F and G 3 days following immunization of the mice. Elevated serum levels of IL-10 were also seen in groups C and D groups on days 14, 28 and 56 after E/SA injection, while in group B, IL-10 serum levels increased following 14 and 28 days but decreased to control levels on day 56 after the immunization of mice (Table 1). The results also demonstrated that IL-10 serum levels were significantly up-regulated in groups C and D after days 14, 28 and 56 compared to groups A and H. (Table 1 & Fig. 1)
Table 1: Serum levels of IL-10 in the designated groups.
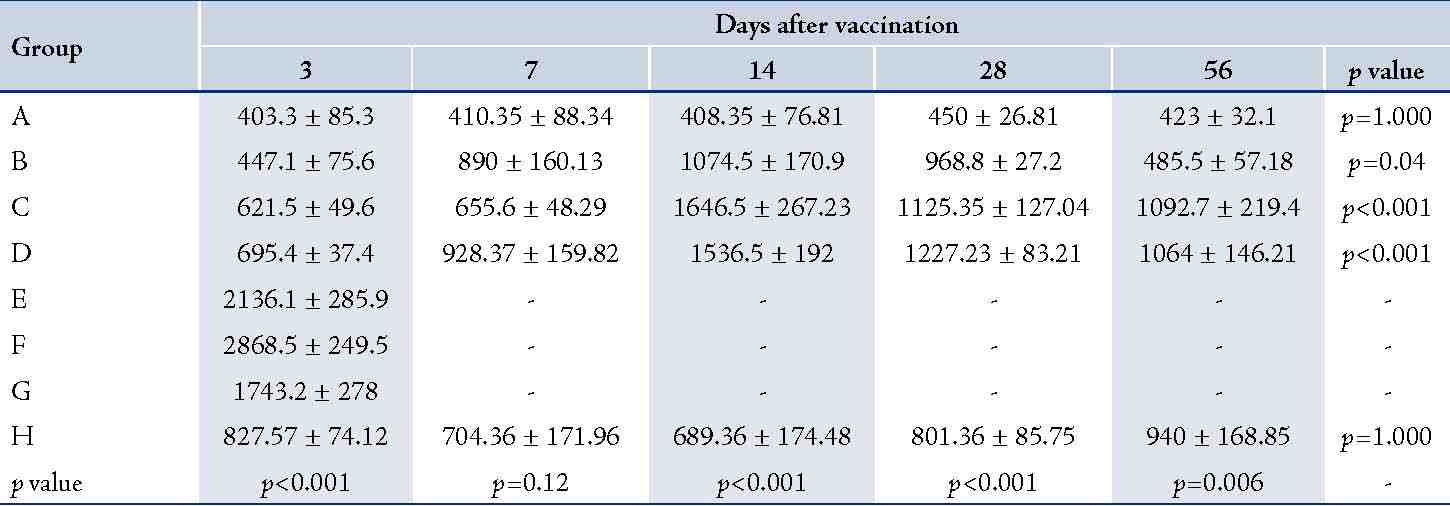
Table shows significant differences between groups after days 14, 28 and 56 after vaccination. Data are shown as mean ± SE (pg/ml),
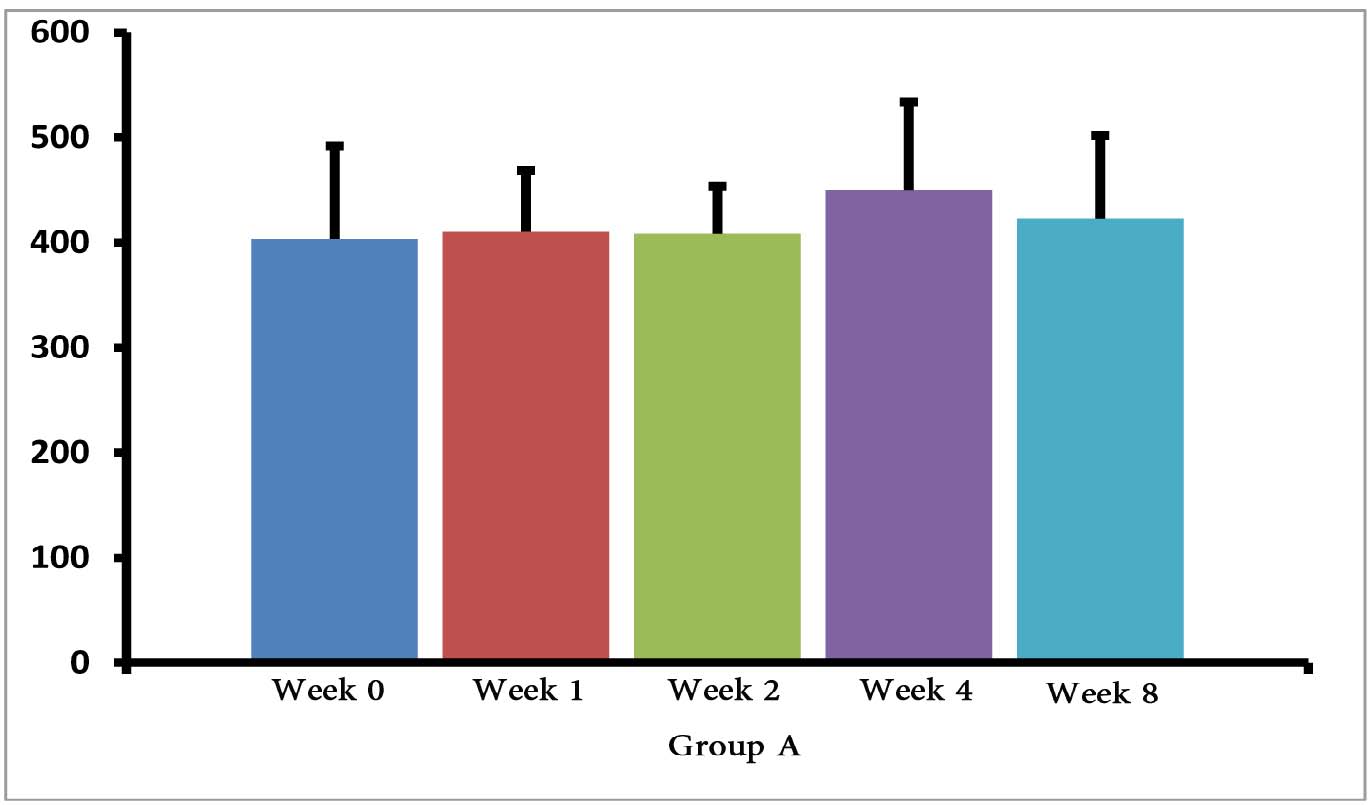 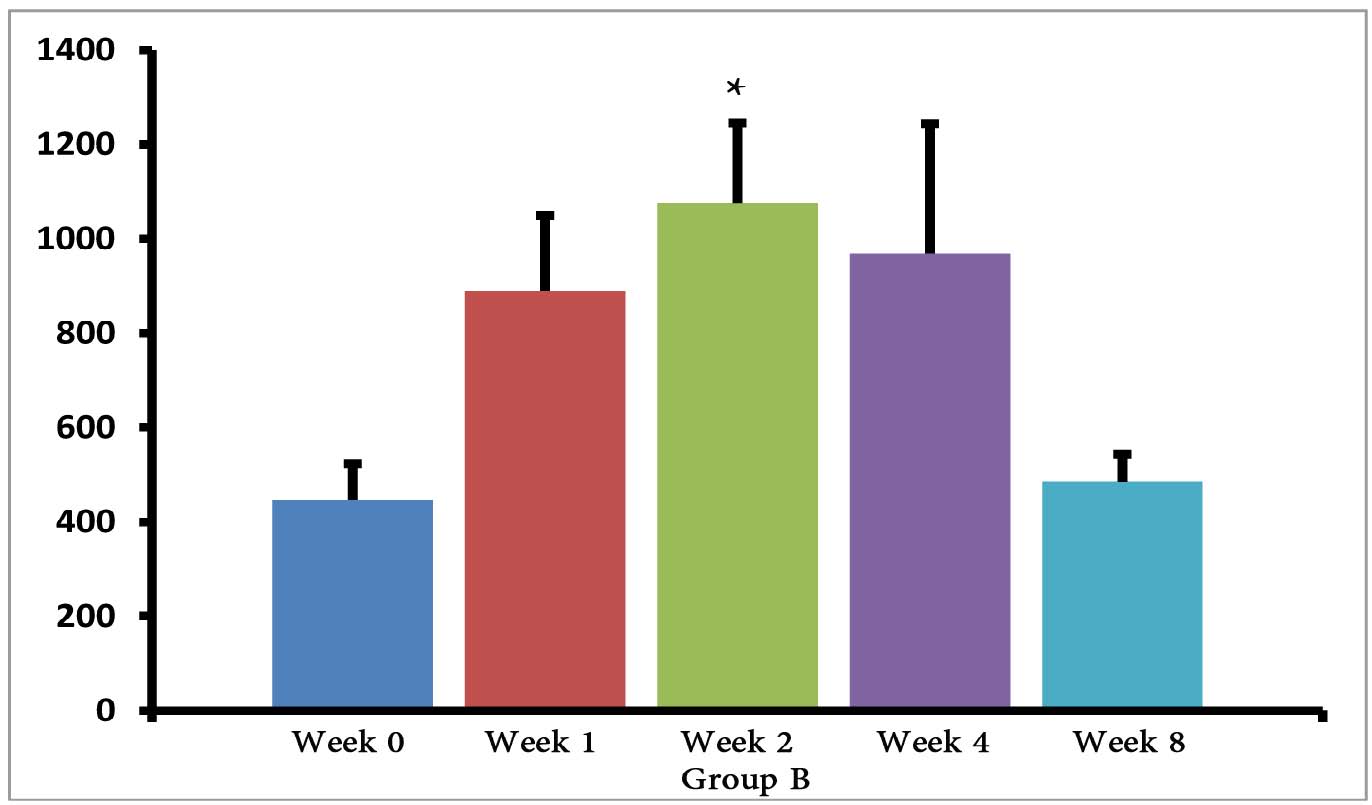 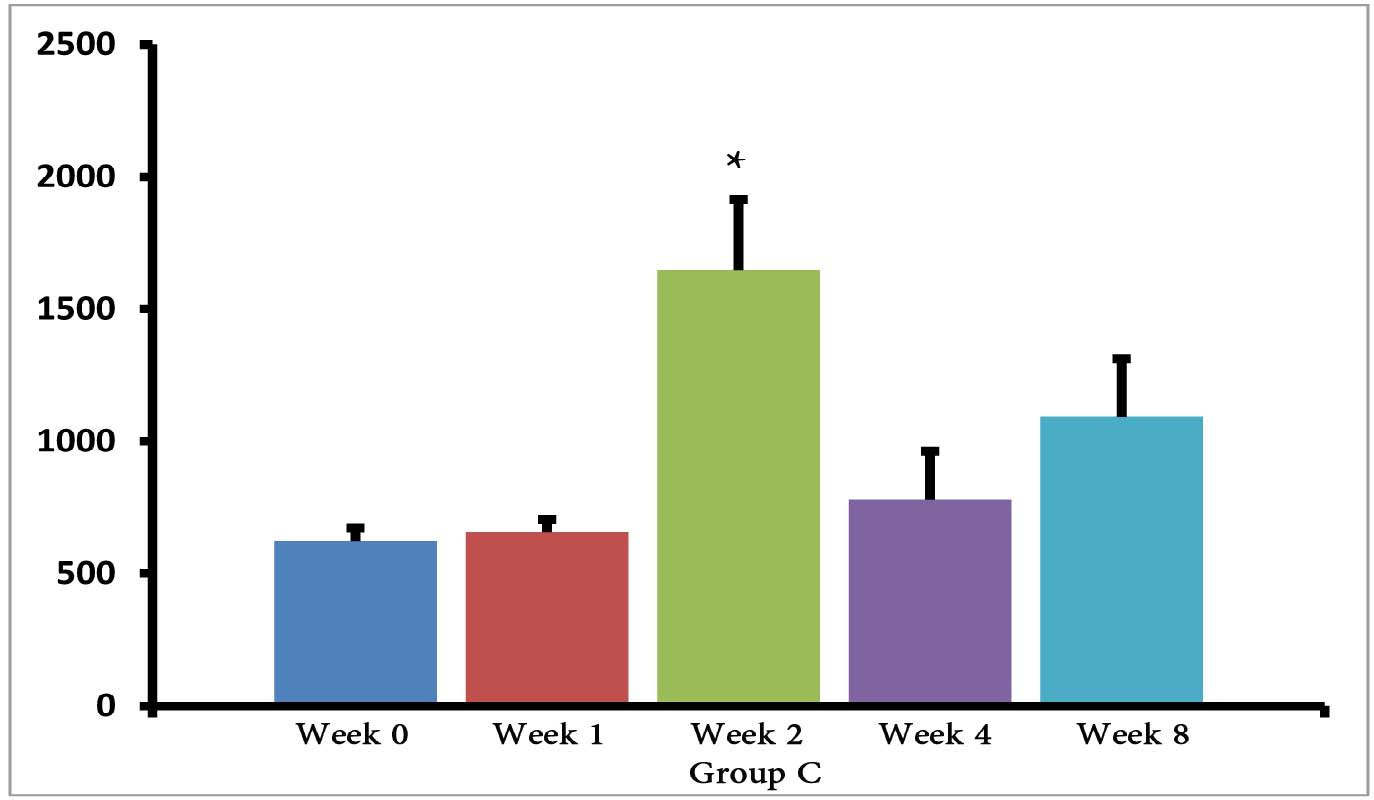 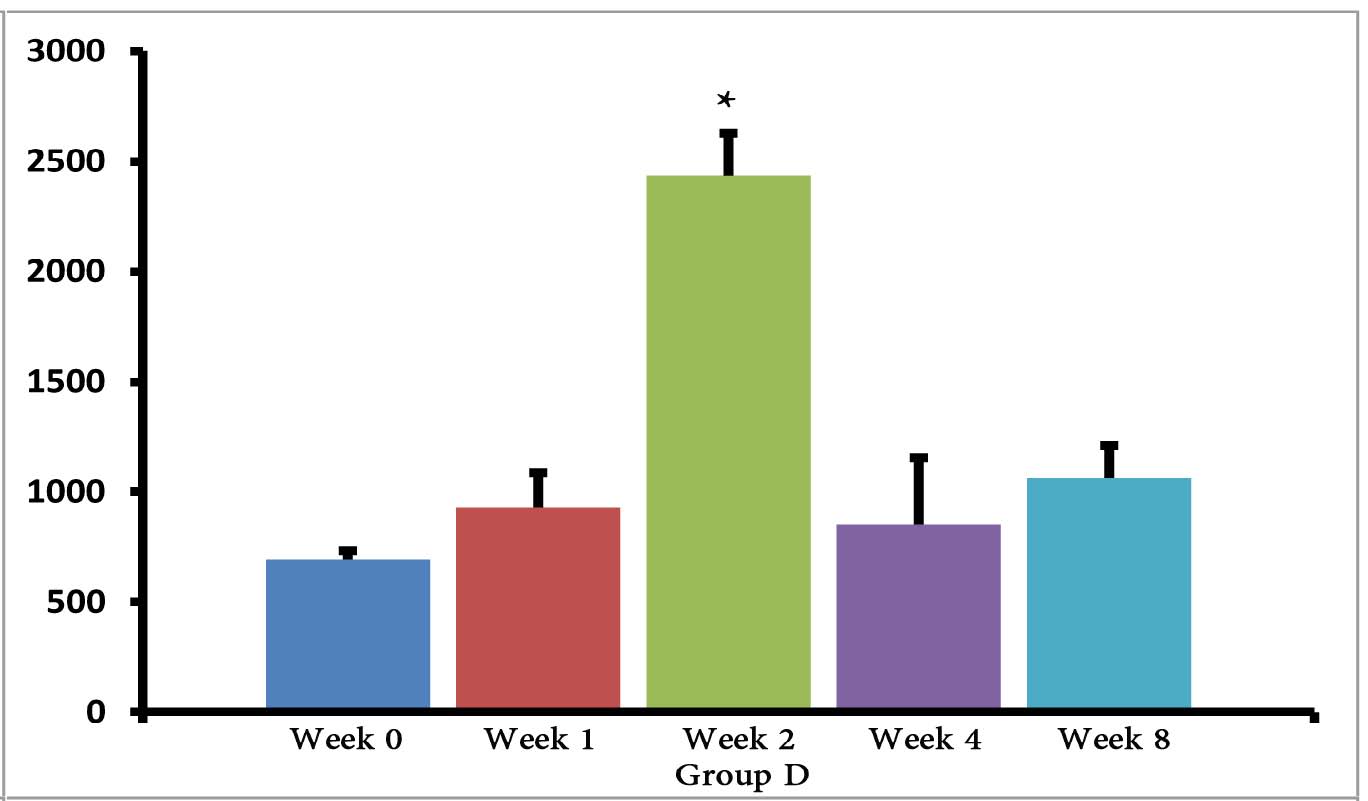 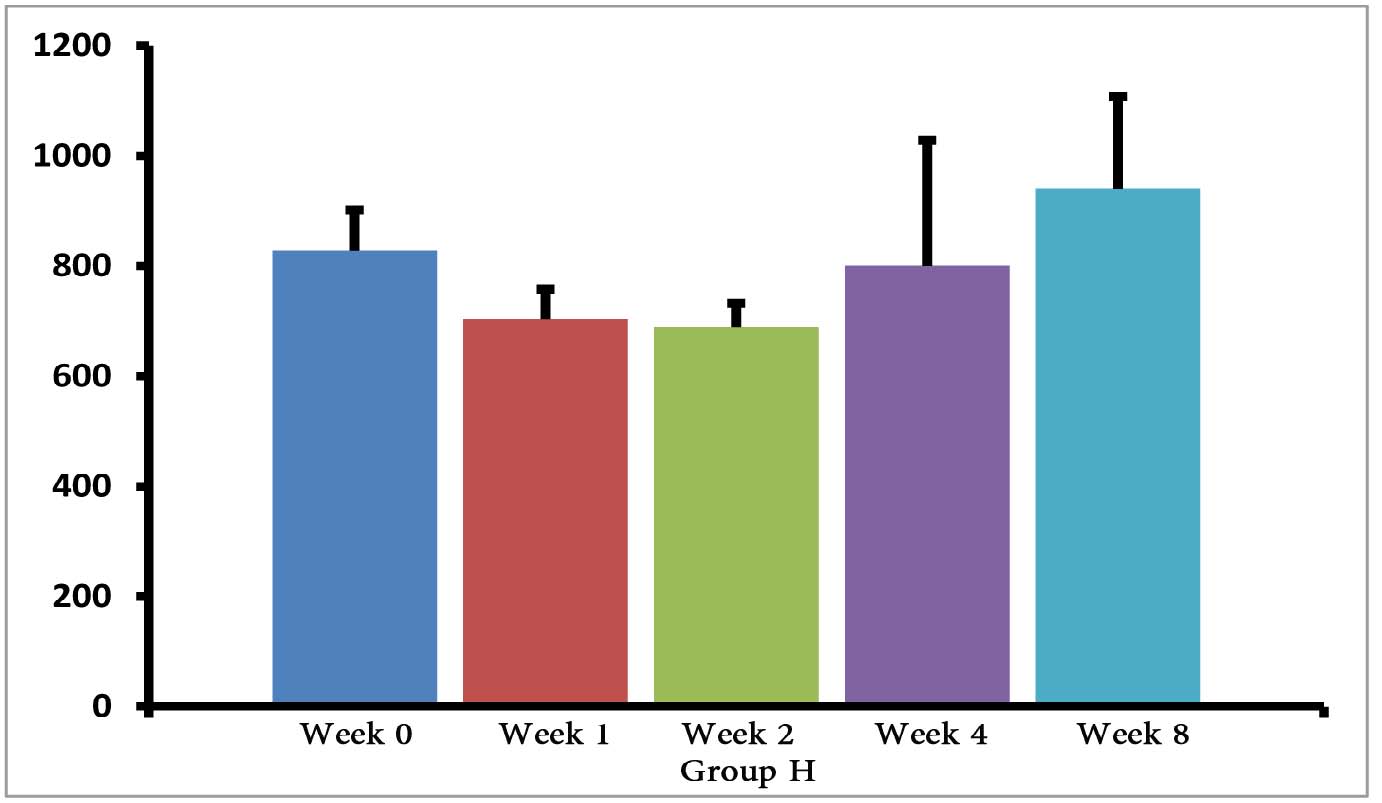
Figure 1: Serum levels of IL-10 in groups B, C and D.
*=significant differences between groups (p<0.001).
Discussion
Toxoplasmosis is a common parasitic infection that is transferred from animals to humans.2 Therefore, the development of a good vaccine is essential for stopping the life circle of Toxoplasma gondii in the animal. To the best of our knowledge, the current study is the first reported investigation based on in vivo prepared E/SA used as a vaccine against T. gondii.
Our data shows that 3 days following the immunization of mice, the serum levels of IL-10 were increased only in the groups of mice infected with T. gondii (E, F and G groups). Previous studies demonstrated that T. gondii induces up-regulation of IL-10 serum levels in order to suppress immune responses of the host.15 Hence, the results demonstrating elevated IL-10 serum levels, as well as death of mice in groups E, F and G, suggest that vaccination with in vivo prepared T. gondii E/SA is unable to suppress the infection. On the other hand, there were no differences observed in serum levels of IL-10 between groups of uninfected mice 3 days following immunization (groups B, C and D); thus, suggesting that at the early phases of vaccination with T. gondii E/SA, there was no change in the serum levels of IL-10 in the mice.
However, IL-10 serum levels were elevated in groups C and D groups on days 14, 28 and 56 following the immunization of mice. While in group B, serum levels of IL-10 increased on days 14 and 28 after the mice were immunized, but the levels returned to control levels on day 56. In groups B, C and D, the elevated serum levels of IL-10 14 or more days after immunization represent the normal immune response in order to induce homeostasis. Namely, the increased IL-10 serum levels suppress elevated immune responses against T. gondii E/SA or following completed Freunds’ adjuvant injection, in order to prevent or to inhibit hypersensitivity of the immune system and autoimmunity processes. Interestingly, our previous study showed that like IL-10 serum levels, the serum levels of TGF-β (another anti-inflammatory cytokine) were also unchanged during the early phases following in vivo prepared T. gondii E/SA injection, although IL-10 levels in the serum of mice increased at the later phases.
Although serum levels of the cytokine IL-10 were high on day 56 in groups C and D of mice receiving completed Freunds’ adjuvant, they decreased to the normal range in the mice that were not injected with completed Freunds’ adjuvant (B group). Hence, these results suggest that the return of IL-10 serum levels from elevated to the control levels on day 56 may be due to the absence of completed Freund’s adjuvant.
Rosenberg et al. showed that the serum levels of IL-10 in mice were increased following DNA vaccination with recombinant chimeric tachyzoite antigens.16 Moreover, increased serum levels of IL-10 were demonstrated 23 days after vaccination of mice against T. gondii with Mic1-3KO tachyzoites.17 On the other hand, DNA vaccine of plasmid encoding the rhoptry protein 1 gene combined with the genetic adjuvant of pcIFN-γ, applied against T. gondii did not induced IL-10 production in mice after 30, 50 and 70 days.18 These results suggest that this vaccination probably produced immune hypersensitivity or autoimmunity in the mice. Some studies which have used other types of the vaccination, including DNA vaccine,19-21 purified protein,22 and subunit vaccines,22 against T. gondii have demonstrated increases in the inflammatory and decreases in the anti-inflammatory cytokines.19-23 Additionally, based on the fact that so many tests were done in the analysis, hence, the multiple testing was as a limitation to discuss the results.
Conclusion
The results of this study and our previous study,2 suggest that in vivo prepared T. gondii E/SA can be considered as a potentially good vaccine for the animals, due to a good anti-E/SA induction, no enhancement of IL-10 serum levels at the early phases, as well as the increase in the serum levels of IL-10 during the final phases of vaccination. Although the advantages of T. gondii E/SA prepared in vivo include the induction of anti-E/SA with higher avidity, as well as the preparation being cheaper and easier E/SA in the mice peritoneum; more aspects of this method of vaccination, such as cellular immunity and animal survival need to be evaluated in order to overcome the mechanisms used by T. gondii to escape from the host immune responses.
Acknowledgements
The authors of this article would like to take this opportunity to thank all the staff at the Molecular and Medicine Research Center and of the animal house at Rafsanjan University of Medical Sciences, who warmly helped this research project. This project was supported by a grant from Rafsanjan University of Medical Sciences.
References
1. Rinaldi L, Scala A. Toxoplasmosis in livestock in Italy: an epidemiological update. Parassitologia 2008 Jun;50(1-2):59-61.
2. Abdollahi SO, Arababadi MK, Hassanshahi G. Evaluation of excreted/secreted antigens derived from peritoneal of toxoplasma infected small mice to detect IgG against Toxoplasma. Pak J Biol Sci 2009 Mar;12(6):530-533.
3. Nissapatorn V. Toxoplasmosis in HIV/AIDS: a living legacy. Southeast Asian J Trop Med Public Health 2009 Nov;40(6):1158-1178.
4. Hide G, Morley EK, Hughes JM, Gerwash O, Elmahaishi MS, Elmahaishi KH, et al. Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology 2009 Dec;136(14):1877-1885.
5. Blokzijl ML. Human immunodeficiency virus infection in childhood. Ann Trop Paediatr 1988 Mar;8(1):1-17.
6. Angus CW, Klivington-Evans D, Dubey JP, Kovacs JA. Immunization with a DNA plasmid encoding the SAG1 (P30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J Infect Dis 2000 Jan;181(1):317-324.
7. El-Malky M, Shaohong L, Kumagai T, Yabu Y, Noureldin MS, Saudy N, et al. Protective effect of vaccination with Toxoplasma lysate antigen and CpG as an adjuvant against Toxoplasma gondii in susceptible C57BL/6 mice. Microbiol Immunol 2005;49(7):639-646.
8. Liu KY, Zhang DB, Wei QK, Li J, Li GP, Yu JZ. Biological role of surface Toxoplasma gondii antigen in development of vaccine. World J Gastroenterol 2006 Apr;12(15):2363-2368.
9. Jongert E, Roberts CW, Gargano N, Förster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz 2009 Mar;104(2):252-266.
10. Daryani A, Hosseini AZ, Dalimi A. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet Parasitol 2003 Apr;113(2):123-134.
11. Darcy F, Deslee D, Santoro F, Charif H, Auriault C, Decoster A, et al. Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol 1988 Sep;10(5):553-567.
12. Odenthal-Schnittler M, Tomavo S, Becker D, Dubremetz JF, Schwarz RT. Evidence for N-linked glycosylation in Toxoplasma gondii. Biochem J 1993 May;291(Pt 3):713-721.
13. Arababadi MK, Mosavi R, Khorramdelazad H, Yaghini N, Zarandi ER, Araste M, et al. Cytokine patterns after therapy with Avonex®, Rebif®, Betaferon® and CinnoVex® in relapsing-remitting multiple sclerosis in Iranian patients. Biomark Med 2010 Oct;4(5):755-759.
14. Vouldoukis I, Mazier D, Moynet D, Thiolat D, Malvy D, Mossalayi MD. IgE mediates killing of intracellular Toxoplasma gondii by human macrophages through CD23-dependent, interleukin-10 sensitive pathway. PLoS One 2011;6(4):e18289.
15. Matowicka-Karna J, Dymicka-Piekarska V, Kemona H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? Clin Dev Immunol, 2009; 2009:374696.
16. Rosenberg C, De Craeye S, Jongert E, Gargano N, Beghetto E, Del Porto P, et al. Induction of partial protection against infection with Toxoplasma gondii genotype II by DNA vaccination with recombinant chimeric tachyzoite antigens. Vaccine 2009 Apr;27(18):2489-2498.
17. Ismael AB, Dimier-Poisson I, Lebrun M, Dubremetz JF, Bout D, Mevelec MN. Mic1-3 knockout of Toxoplasma gondii is a successful vaccine against chronic and congenital toxoplasmosis in mice. J Infect Dis 2006 Oct;194(8):1176-1183.
18. Guo H, Chen G, Lu F, Chen H, Zheng H. Immunity induced by DNA vaccine of plasmid encoding the rhoptry protein 1 gene combined with the genetic adjuvant of pcIFN-gamma against Toxoplasma gondii in mice. Chin Med J (Engl) 2001 Mar;114(3):317-320.
19. Makino M, Uemura N, Moroda M, Kikumura A, Piao LX, Mohamed RM, et al. Innate immunity in DNA vaccine with Toxoplasma gondii-heat shock protein 70 gene that induces DC activation and Th1 polarization. Vaccine 2011 Feb;29(10):1899-1905.
20. Yan HK, Yuan ZG, Petersen E, Zhang XX, Zhou DH, Liu Q, et al. Toxoplasma gondii: protective immunity against experimental toxoplasmosis induced by a DNA vaccine encoding the perforin-like protein 1. Exp Parasitol 2011 May;128(1):38-43.
21. Yuan ZG, Zhang XX, He XH, Petersen E, Zhou DH, He Y, et al. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin Vaccine Immunol 2011 Jan;18(1):119-124.
22. Dziadek B, Gatkowska J, Brzostek A, Dziadek J, Dzitko K, Grzybowski M, et al. Evaluation of three recombinant multi-antigenic vaccines composed of surface and secretory antigens of Toxoplasma gondii in murine models of experimental toxoplasmosis. Vaccine 2011 Jan;29(4):821-830.
23. Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, et al. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine 2011 Jan;29(4):754-762.
|
|