| |
Abstract
Objective: The relationship between parasites and pediatric appendicitis is a highly debatable issue. This study aims to investigate the role of parasitic infestation in the etiology of acute pediatric appendicitis.
Methods: A retrospective study including 1600 pediatric and adolescent patients who had undergone surgical therapy for a diagnosis of acute appendicitis over a period of ten years from Jan 2001 to Dec 2010. Demographic data were retrieved including the patient's age, sex, clinical data, clinical presentations, laboratory investigations, operative data and pathological findings to identify the presence and type of parasites. Patients were divided into two groups according to the presence or absence of parasites in the appendix lumen. In group I (n: 88), parasitic infestation was observed, whereas in group II (n: 1502), no parasitic infestation was present.
Results: Parasites were present in 5.5% (88 patients), and of those 88 parasitic infestations, 45 (51.1%) were Enterobaisis, 8 (9.1%) were Schistosomiasis, 23 (26.1%) were Ascariasis, 7 (8%) Trichuriasis, and 5 (5.7%) were Teania Saginata. The percentage of patients with suppurative, gangrenous or perforated appendicitis was similar in both groups with no statistical significance, irrespective of the presence or absence of parasitic infestation.
Conclusion: The low prevalence of parasites among the appendectomy specimens did not support the notion that parasites were a major cause of appendicitis in pediatric patients.
Keywords: Appendicitis; Parasitic infestation; Enterobiasis; Al-Ahsa.
Introduction
Acute appendicitis is the most common pathological cause of appendectomy; however, various other pathological entities are found in children. Among those factors, parasites may play a considerable role.1-3 It has been documented that Enterobius vermicularis (pinworm) infections of the gastrointestinal tract occur in 4-28% of children worldwide, with a high prevalence in developing and tropical countries.4-9 Although the most common manifestation of pinworms is the perianal pruritus, pinworms have been found in multiple other locations, including the appendix.10-14
Recent literature on appendiceal parasites focuses primarily on the pathological changes induced by the presence of intraluminal parasites. The current retrospective study aimed to assess the role of parasitic infestation in the etiology of pediatric appendicitis.
Methods
This is a retrospective study including the medical records of all pediatric and adolescent patients who were admitted and surgically treated for acute appendicitis from Jan 2001 to Dec 2010 (n: 1600 patients). The ethical considerations were fulfilled and data was confidentially maintained throughout the study. The patients’ records were thoroughly reviewed to gather the demographic data such as age, sex and residence. Clinical data including the chief complaints on admission, duration of symptoms, history of similar attacks, previous hospitalization and history of any chronic diseases plus drug history (if any) were analyzed. Laboratory and imaging studies were also reviewed.
The data from surgical procedures were collected in terms of a thorough study of operative data and the descriptive review of the operative findings during surgery, as well as the duration of surgery were all reported. The presence of postoperative complications was analyzed. Collected data were coded, entered and statistically analyzed using SPSS version 16. Two tailed tests of significance were used with 95% confidence level. Continuous data were tested for their distribution assumption. Categorical variables were expressed as frequency and percentage. Chi-square and Z-test were used for comparison wherever appropriate. For continuous variables, the mean and standard deviations were used for reporting the data, while the Kruskal Wallis test was used for comparing the means of the different groups. A p value ≤0.05 was considered significant.
Results
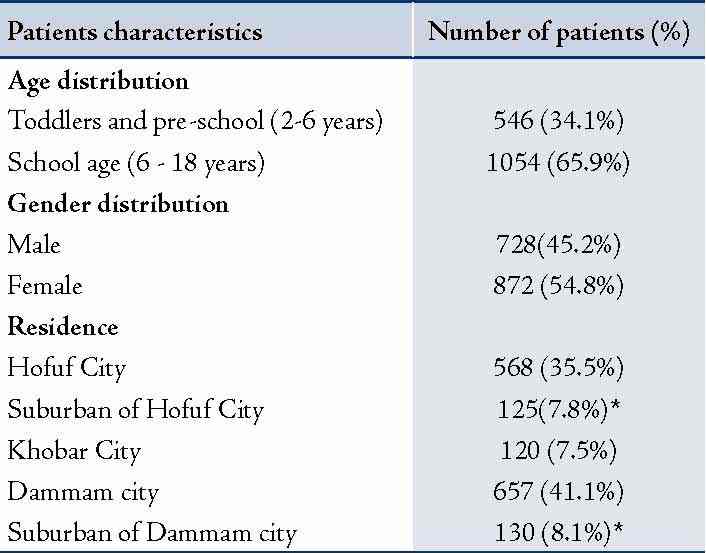
Patients were divided into two groups according to the presence or absence of parasites in the appendix lumen: In group I (n: 88), parasitic infestation was observed; whereas, in group II (n: 1502), no parasitic infestation was present. Out of the total number, 872 (54.5%) were females and 728 (45.5%) were males with F/M ratio of 1.2:1. Most patients with parasitic infestations were living or lived in rural or suburban areas compared to those living in urban areas (p<0.03). (Table 1)
Table 1: Demographic data of the studied patients.
One-thousand four-hundred and nineteen patients (88.7%) complained of lower right quadrant abdominal pain. The remaining patients complained of pain associated with diarrhea which was reported in 99 patients (54.7%), 34 (18.8%) patients had associated vomiting and fever, while 48 (26.5%) suffered from associated attacks of constipation preceded by diarrhea.
Surgical procedures were open appendectomy in 1480 patients (92.5%) and laparoscopic surgery in 120 patients (7.5%). The operative findings ranged from acute edematus congested appendix in 1344 patients (84%), apparently normal in 139 patients (8.7%), perforated in 35 patients (2.2%), obstructed in 43 patients (2.7%), and 39 (2.4%) had appendicitis which were gangrenous. The operative time ranged from 22 minutes to 94 minutes with a median of 31 minutes.
Associated intra-abdominal diseases included Meckel's diverticulum in 6 patients (0.4%), perforation of the appendix with Ascaris worm seen in the peritoneal cavity of 3 patients (0.2%), and 4 (0.3%) pinworm perforations of the appendix, as well as ovarian cyst torsion in 4 patients (0.3%). Postoperative complications included pelvic collection in 8 patients (0.5%), wound infection and dehiscence in 37 patients (2.3%), and postoperative chest infection in 3 patients (0.2%).
In 584 (36.5%) specimens, the appendix showed an acute catarrhal inflammation. Eosinophil infiltration was microscopically seen in 53 specimens that also showed different parasitic infiltration, while neutrophil infiltration was noted in the majority of specimen. In addition, among 725 (45.3%) of the specimen, acute diffuse suppuration of the appendix was recorded of whom 33 specimens had different parasitic and eosinophilic infiltrations which were recorded. (Table 2)
Table 2: Histopathological and laboratory findings. (n=1600)
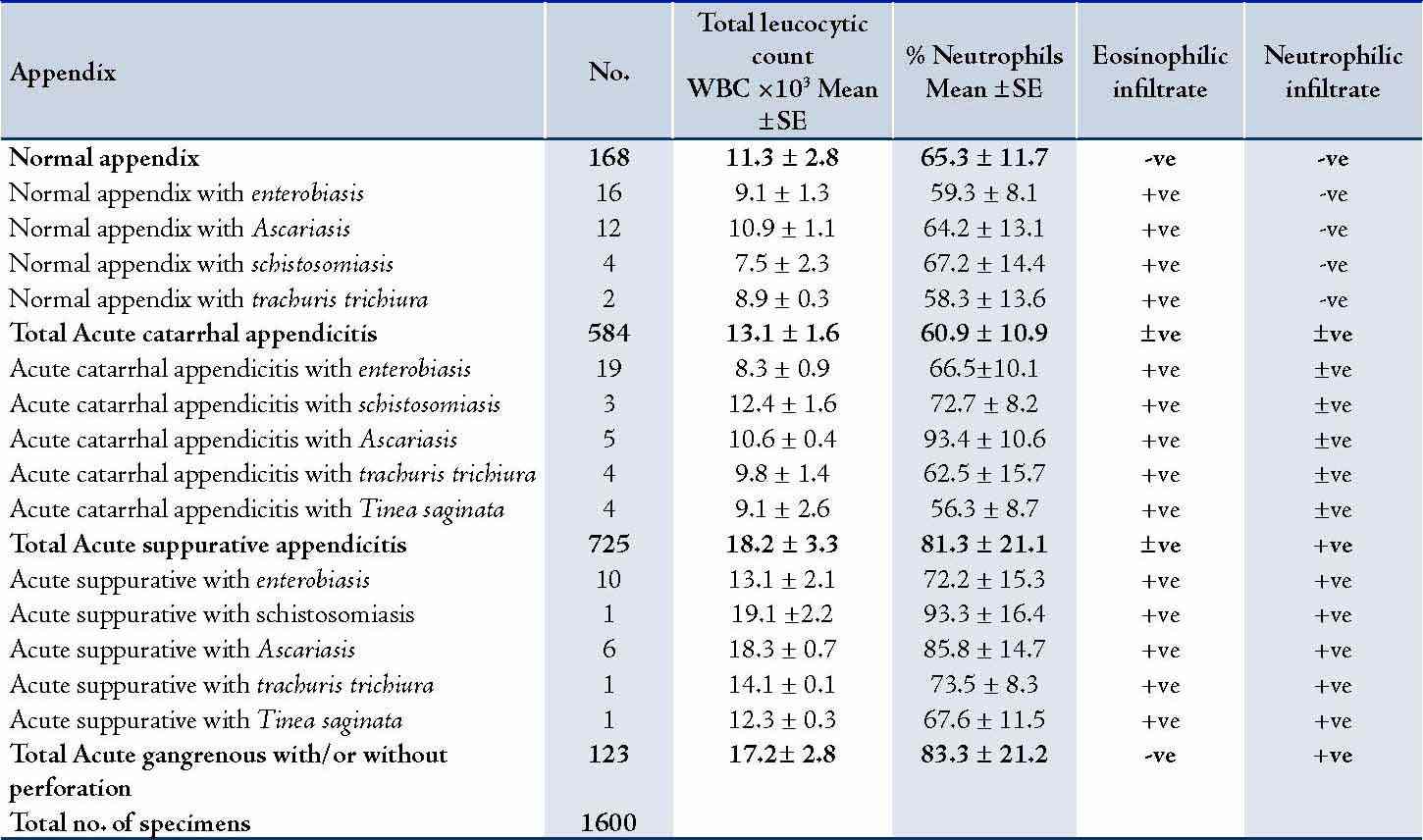
+ve: present in all specimens; -ve: Absent in all specimens; ±ve: Present in some specimens and absent in others.
The appendix was seen to be normal in 168 (10.5%) of the studied specimens; and in 123 (7.7%) specimens, the appendix was acutely gangrenous. A total of 54 (3.8%) specimens showed parasitic infestation associated with histopathologically proven acute appendicitis. However in 34 (2.1%) specimens, there was parasitic infestation in histopathologically normal appendix. (Table 3)
Table 3: Distribution of Parasitic infestation in the studied patients.

Comparison of the prevalence rates of parasitic infestation between appendicitis cases and normal appendix showed that except for Teania saginata, the prevalence rate was significantly higher among the normal appendix than the Appendicitis group for every single species as well as for total infestation (p<0.01). The results also showed that the prevalence of Trichuriasis was still higher among the normal appendices (group II), which was almost significant. (Table 4)
Discussion
Gastrointestinal infection due to parasitic infestation occurs worldwide. It has been a controversial issue in terms of its role in the etiology of acute appendicitis. Pinworm is considered the most common helminthes infection.15 It has been postulated that pinworm infestation with acute appendicitis varies from 0.2% to 41.8% worldwide.3 Although seen in all ages and socioeconomic levels, it is most common in children aged from 5 to 14 years. It did appear in 2.8% of the current studied subjects, this could be compared to the figures reported in the literature of 1549 appendectomies, in which 1.4% specimens were found to contain pinworm.3 It also does coincide with other published data,2 where parasites were present in 62 (7.5%) cases out of a total 830 appendectomy specimens. In a wide literature review of 21 publications for appendiceal enterobiasis infections for the period from 1957 to 2002, appendiceal pinworm represented approximately 4.5% in appendectomies.3 (Table 5)
Table 4: Comparison of the prevalence Rates of Parasitic Infestation between Appendicitis cases and Normal Appendix.
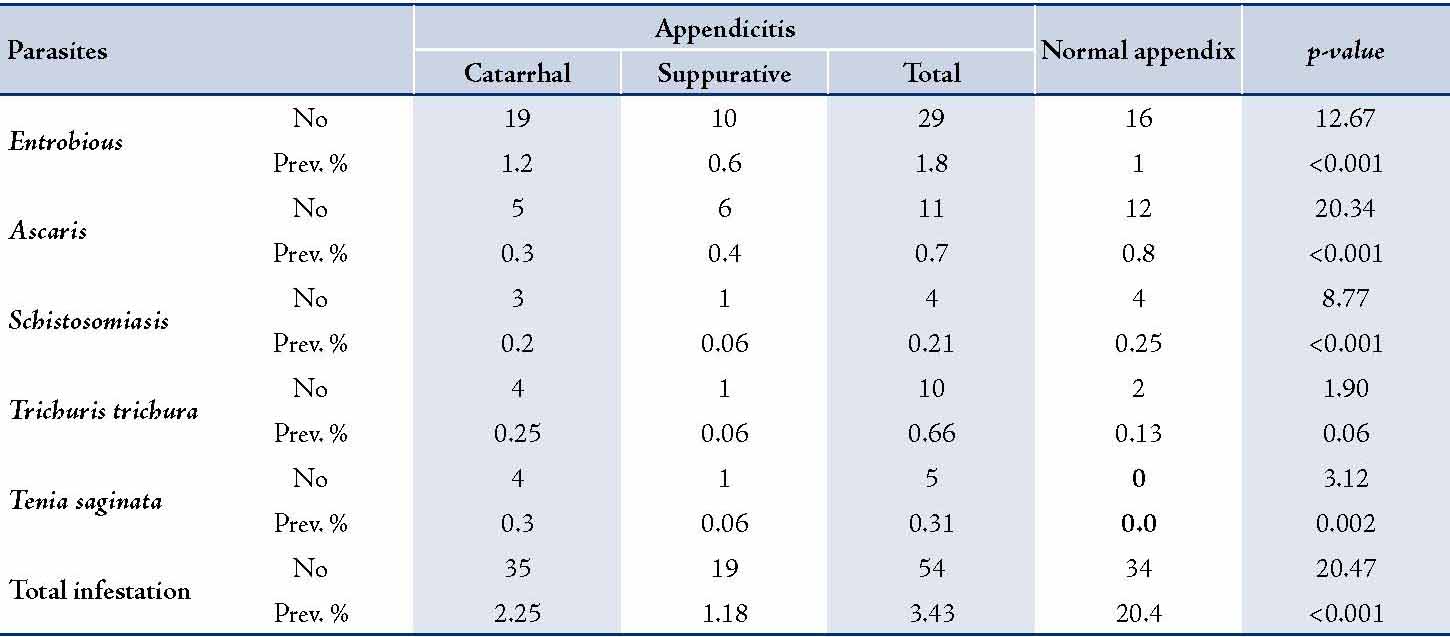
Table 5: Literature review of appendiceal enterobius infections.3
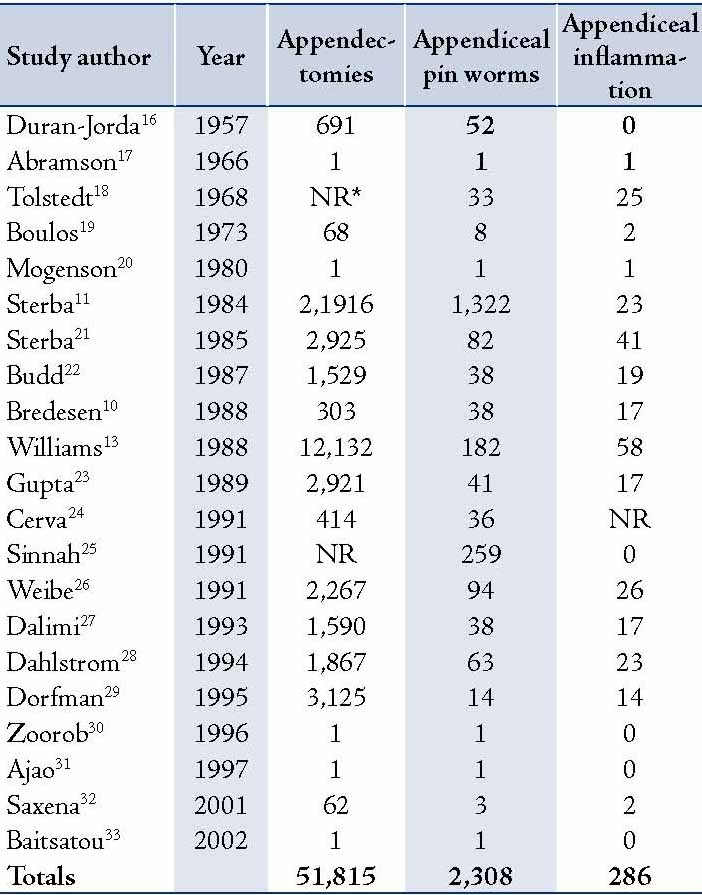
* (NR= not reported)
We hereby reported an incidence of normal appendices with parasitic infestation coinciding with similarly published data by the author and others.29,34 Pinworm was more often associated with un-inflamed appendices than inflamed appendices, and mucosal invasion was not seen; therefore, it would seem unlikely that these parasites cause acute appendicitis. However, pinworm may be a cause of symptoms resembling appendicitis, because a significantly higher proportion of patients with symptoms had pinworm compared with patients who had an incidental appendicectomy.1-3
Schistosomas were seen in 0.5% in our studied specimens, this coincides with some published data that showed the percentage of schistosomal appendicitis to be 1.6%.35 Moreover, it has been recorded to be only 0.2% in Hong Kong.36 Some others reported different percentages of schistosomal appendicitis recorded as 2.3% and 1.3%, respectively.37,38
Parasitic infestation is endemic within Saudi Arabia, especially in rural areas, as with other developing countries. The results of this study reflect this natural prevalence of the disease. These results however, contradict previously published data by the same author on Egyptian patients, where such incidence was higher in urban areas.34
The cause of acute appendicitis is generally considered to be an obstruction at the base of the appendix. An appendix affected by Schistosomiasis would show considerable fibrosis that may have led to the obstruction.38 The patients with Schistosomas present in histopathological specimen were symptomatic in 4 out of 8 (50%). These symptoms were not classical for acute appendicitis; the reason for that could be explained on the basis of low grade chronic inflammation solely due to Schistosomiasis. Moreover, the fibrosis associated with schistosomal appendicitis that may limit the spread of local inflammation may explain the low leucocyte count in that group.39
In the current study, Ascaris lumbricoids and Trichuris trichura were the encountered parasites in 1.4% and 0.4%, respectively, in appendiceal specimens. This can be compared to previously published data.29,34
Laparoscopy was performed as a diagnostic and therapeutic maneuver in 113 out of the total 1500 patients. It showed free pinworms in 3 patients and also free Ascaris in the abdominal cavity of one patient. Nevertheless, laparotomy showed free pinworms in the peritoneal cavity due to appendiceal perforation in one patient. The data coincides with a literature report of 3 cases in which pinworms were set free into the abdominal cavity during laparoscopic appendectomy. It has been recommended that surgeons should exercise caution when performing laparoscopic appendectomy, using the endo-loop technique to ensure that the pinworms are not released into the peritoneum upon amputation of the appendix.32
In this study, parasitic infestation of the appendix was thought to play a role in causing disease states including acute appendicitis, chronic appendicitis and appendicial perforation, especially in cases of Enterobiasis and Ascariasis infection. These parasitic infestations however, have also been found in symptom-free patients. It is imperative that patients with parasitic infestation receive the appropriate antiparasitic treatment before being discharged from hospital, because the appendectomy is treated as a consequence and not the root of the disease.
Conclusion
It can be postulated that the low prevalence of parasites among appendectomy specimen may support the idea that parasitic worms may be a minor or coincidental cause of pediatric appendicitis, thus refuting the hypothesis that parasites may be a major cause of pediatric appendicitis in parasite endemic areas.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Karatepe O, Adas G, Tukenmez M, Battal M, Altiok M, Karahan S. Parasitic infestation as cause of acute appendicitis. G Chir 2009 Oct;30(10):426-428.
2. Dorfman S, Cardozo J, Dorfman D, Del Villar A. The role of parasites in acute appendicitis of pediatric patients. Invest Clin 2003 Dec;44(4):337-340.
3. Arca MJ, Gates RL, Groner JI, Hammond S, Caniano DA. Clinical manifestations of appendiceal pinworms in children: an institutional experience and a review of the literature. Pediatr Surg Int 2004 May;20(5):372-375.
4. Gatti S, Lopes R, Cevini C, Ijaoba B, Bruno A, Bernuzzi AM, et al. Intestinal parasitic infections in an institution for the mentally retarded. Ann Trop Med Parasitol 2000 Jul;94(5):453-460.
5. Henley M, Sears JR. Pinworms: a persistent pediatric problem. MCN Am J Matern Child Nurs 1985 Mar-Apr;10(2):111-113.
6. Lee DS, Chung BH, Lee NS, Nam HW, Kim JH. A survey of helminthic infections in the residents of rural areas near Ulaanbaatar, Mongolia. Korean J Parasitol 1999 Sep;37(3):145-147.
7. Liu LX, Weller PF. Intestinal nematodes. In: Isselbacher KJ. Braunwald E (eds) Harison's principles of internal medicine, 13th edn. McGraw-Hill, New York, p 919.
8. Yoon HJ, Choi YJ, Lee SU, Park HY, Huh S, Yang YS. Enterobius vermicularis egg positive rate of pre-school children in Chunchon, Korea (1999). Korean J Parasitol 2000 Dec;38(4):279-281.
9. Ball MT, Hay J. Simultaneous demonstration of eosinophilic granulocytes and mast cells in tissue sections containing helminths. Ann Trop Med Parasitol 1990 Apr;84(2):195-196.
10. Bredesen J, Falensteen Lauritzen A, Kristiansen VB, Sørensen C, Kjersgaard P. Appendicitis and enterobiasis in children. Acta Chir Scand 1988 Oct;154(10):585-587.
11. Stĕrba J, Vlcek M. Appendiceal enterobiasis–its incidence and relationships to appendicitis. Folia Parasitol (Praha) 1984;31(4):311-318.
12. Still GF. Observations on oxyuris vermicularis in children. Br Med J. 1899 Apr 15;1(1998):898-900.
13. Williams DJ, Dixon MF. Sex, Enterobius vermicularis and the appendix. Br J Surg 1988 Dec;75(12):1225-1226.
14. Sridhar R, Kapila K, Verma K. Cytologic diagnosis of Enterobius vermicularis eggs in an enterocutaneous fistula. Indian J Pathol Microbiol 1999 Jul;42(3):355-357.
15. Symmers WS. Pathology of oxyuriasis; with special reference to granulomas due to the presence of Oxyuris vermicularis (Enterobius vermicularis) and its ova in the tissues. AMA Arch Pathol 1950 Oct;50(4):475-516.
16. Duran-Jorda F. Appendicitis and enterobiasis in children; a histological study of 691 appendices. Arch Dis Child 1957 Jun;32(163):208-215.
17. Abramson DJ. Acute appendicitis and a Meckel’s diverticulum with Enterobius vermicularis. First reported case. Am Surg 1966 May;32(5):343-346.
18. Tolstedt GE. Pinworm infestation of the appendix. Am J Surg 1968 Sep;116(3):454-455.
19. Boulos PB, Cowie AG. Pinworm infestation of the appendix. Br J Surg 1973 Dec;60(12):975-976.
20. Mogensen K, Pahle E, Kowalski K. Enterobius vermicularis and acute appendicitis. Acta Chir Scand 1985;151(8):705-707.
21. Stĕrba J, Vlcek M, Noll P, Vorel F. Contribution to the question of relationships between Enterobius vermicularis (L.) and inflammatory processes in the appendix. Folia Parasitol (Praha) 1985;32(3):231-235.
22. Budd JS, Armstrong C. Role of Enterobius vermicularis in the aetiology of appendicitis. Br J Surg 1987 Aug;74(8):748-749.
23. Gupta SC, Gupta AK, Keswani NK, Singh PA, Tripathi AK, Krishna V. Pathology of tropical appendicitis. J Clin Pathol 1989 Nov;42(11):1169-1172.
24. Cerva L, Schrottenbaum M, Kliment V. Intestinal parasites: a study of human appendices. Folia Parasitol (Praha) 1991;38(1):5-9.
25. Sinniah B, Leopairut J, Neafie RC, Connor DH, Voge M. Enterobiasis: a histopathological study of 259 patients. Ann Trop Med Parasitol 1991 Dec;85(6):625-635.
26. Wiebe BM. Appendicitis and Enterobius vermicularis. Scand J Gastroenterol 1991 Mar;26(3):336-338.
27. Dalimi A, Khoshzaban F. Comparative study of two methods for the diagnosis of Enterobius vermicularis in the appendix. J Helminthol 1993 Mar;67(1):85-86.
28. Dahlstrom JE, Macarthur EB. Enterobius vermicularis: a possible cause of symptoms resembling appendicitis. Aust N Z J Surg 1994 Oct;64(10):692-694.
29. Dorfman S, Talbot IC, Torres R, Cardozo J, Sanchez M. Parasitic infestation in acute appendicitis. Ann Trop Med Parasitol 1995 Feb;89(1):99-101.
30. Zoorob RJ. Appendiceal colic caused by Enterobius vermicularis. J Am Board Fam Pract 1996 Jan-Feb;9(1):57-59.
31. Ajao OG, Jastaniah S, Malatani TS, Morad N, el Tayeb EN, Saif SA, et al. Enterobius vermicularis (pin worm) causing symptoms of appendicitis. Trop Doct 1997 Jul;27(3):182-183.
32. Saxena AK, Springer A, Tsokas J, Willital GH. Laparoscopic appendectomy in children with Enterobius vermicularis. Surg Laparosc Endosc Percutan Tech 2001 Aug;11(4):284-286.
33. Batistatou A, Zolota V, Scopa CD. Images in pathology: oxyuris (enterobius) vermicularis in human cecum and appendix. Int J Surg Pathol 2002 Jan;10(1):58.
34. Zakaria O. The controversy of parasitic infection in pediatric appendicitis: a retrospective analysis. Ann Pediatr Surg 2012;8(1):15-18 .
35. Satti MB, Tamimi DM, Al Sohaibani MO, Al Quorain A. Appendicular schistosomiasis: a cause of clinical acute appendicitis? J Clin Pathol 1987 Apr;40(4):424-428.
36. Chan KW, Fu KH, Ling J. The pathology of the appendix in Hong Kong. Br J Clin Pract 1988 Jun;42(6):241-244.
37. Adebamowo CA, Akang EE, Ladipo JK, Ajao OG. Schistosomiasis of the appendix. Br J Surg 1991 Oct;78(10):1219-1221.
38. Abu-Eshy SA, Malik GM, Khan AR, Khan GM, Al-Shehri MY. Schistosomal appendicitis. Ann Saudi Med 1995 Jul;15(4):347-349.
39. Meshikhes AW, Chandrashekar CJ, Al-Daolah Q, Al-Saif O, Al-Joaib AS, Al-Habib SS, et al. Schistosomal appendicitis in the Eastern Province of Saudi Arabia: A clinicopathological study. Ann Saudi Med 1999 Jan-Feb;19(1):12-14.
|
|