|
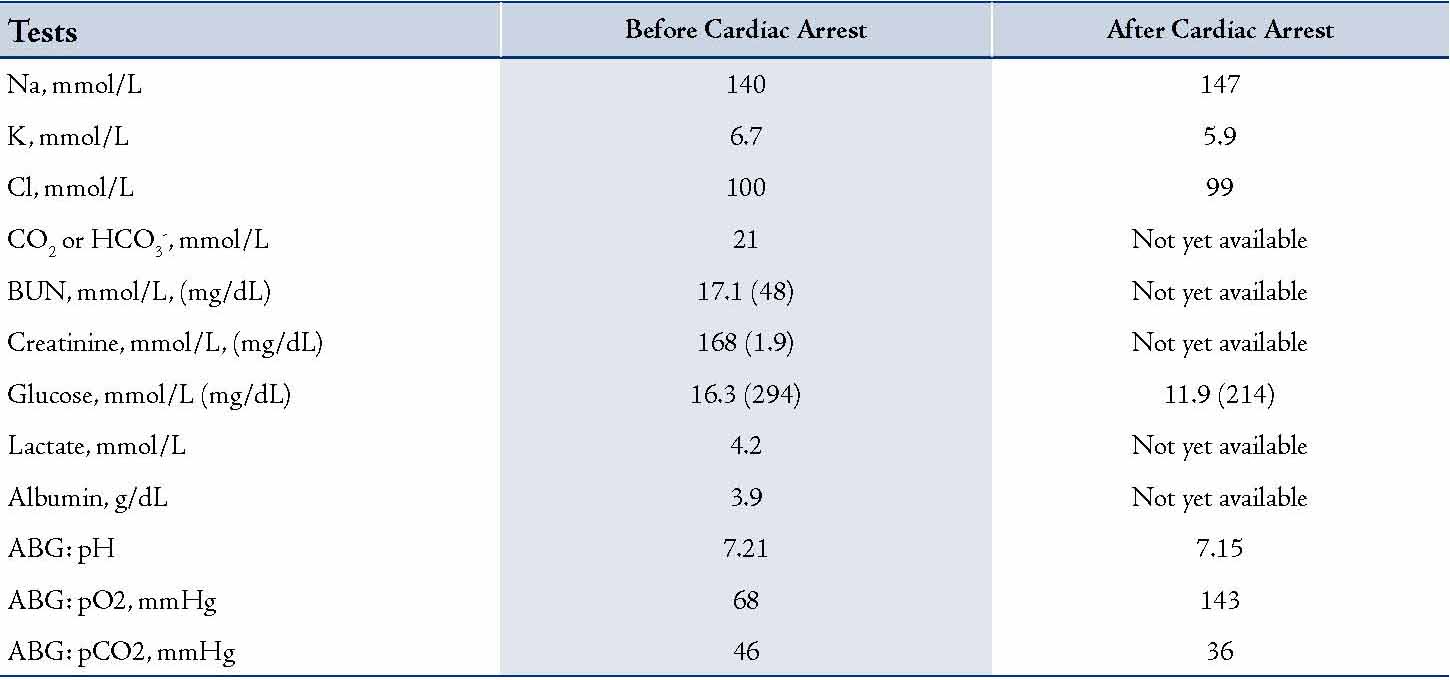
An 82 year-old male patient with past medical history of coronary artery disease, hypertension, COPD, diabetes mellitus (Type 2), chronic kidney disease, Alzheimer’s disease and recently treated diarrhea was brought to the emergency department (ED) from home after he vomited, aspirated and developed severe respiratory distress. He was emergently intubated on arrival to ED. Shortly after intubation, he became hypotensive, bradycardic and went into cardiac arrest. During ACLS, the patient required a total of 17 minutes of CPR and 2 shocks. He received 2 liters of normal saline solution intravenously and multiple doses of epinephrine, sodium bicarbonate and calcium gluconate. He was also started on amiodarone and epinephrine infusions. Blood tests were obtained before the cardiac arrest, with a limited panel collected immediately after the patient regained circulation. These values are shown in Table 1.
Table 1: Blood test results
Question
Based on the available data, what metabolic acid-base disorders are present in this patient after cardiac arrest?
A. Not enough data to evaluate.
B. Anion gap metabolic acidosis and non-anion gap metabolic acidosis
C. Anion gap metabolic acidosis and metabolic alkalosis
D. Anion gap metabolic acidosis only
E. Non-anion gap metabolic acidosis only
Answer
C. Anion gap metabolic acidosis and metabolic alkalosis
Discussion
Under most circumstances, clinicians rely on the serum bicarbonate concentration to evaluate the anion gap and the metabolic component of an acid-base disturbance. This case illustrates how a mixed metabolic acid-base disorder could be evaluated without knowledge of the serum bicarbonate level by using Delta Gap (DG) equation. In patients with known anion gap metabolic acidosis, the value of serum bicarbonate (HCO3-) in the DG equation cancels, thus requiring only two serum values (Na+ and Cl-) to assess concomitant metabolic acid-base disturbances.
Determination of the anion gap (AG) is one of the initial, and very important, steps in the differential diagnosis of acid-base disorders.1 The concept of the AG is based on the following assumptions: A total concentration of anions and cations in plasma is equal, and some of the anions and cations cannot be measured. The difference between the concentration of unmeasured anions and cations can be estimated by calculating the AG representing the difference between the primary measured cations and the primary measured anions. By convention, AG=Na+-(Cl-+ HCO3-). A normal AG primarily reflects the concentration of non-bicarbonate buffers including albumin, phosphate, sulfate, and other organic acids.
Elevated AG usually represents abnormal accumulation of either endogenous or exogenous unmeasured anions and indicates a primary disorder (a metabolic acidosis), regardless of the pH or the serum bicarbonate (HCO3-).2 The substantial increase in unmeasured anions will be accompanied by an equimolar decrease in bicarbonate unless the bicarbonate level is altered by another concomitant metabolic acid-base disturbance. Therefore, when AG metabolic acidosis is diagnosed, it is imperative to screen for the presence of additional acid-base abnormalities (called mixed metabolic acid-base disorders). The equations detailed below can be utilized to assess the presence and nature of mixed acid-base disorders.3
(Delta Ratio)=∆AG/∆HCO3- or (∆∆ or Delta Gap)=∆AG-∆HCO3-
For both formulas: ∆AG(excess anion gap)=AG(actual anion gap)-12(normal anion gap) and ∆HCO3-(bicarbonate deficit)=24(normal bicarbonate level)-HCO3-(actual bicarbonate level). It is well known that by using Henderson equation ([H+] [HCO3-] = k x pCO2) bicarbonate level (HCO3-) can be calculated from known pH and pCO2. However, the equation describes ideal solution and has multiple approximations and assumptions limiting its accuracy in some circumstances, particularly in a clinical setting. Moreover, the blood samples taken from the arterial (for arterial blood gas (ABG) analysis) and venous (for metabolic panel) circulation could produce substantial differences in the values used for calculations.4 Which is why it is recommended to use measured instead of calculated bicarbonate level. Most of ABG analyzers calculate HCO3- from pH and pCO2. In our case we do not have the measured bicarbonate level to use in our calculations. However, the obvious advantage of using the Delta Gap (DG) equation over Delta Ratio is that it can be easily simplified to use with limited data without bicarbonate level determination.
Delta Gap=∆AG-∆HCO3-=Na+-(Cl++HCO3-)-12-(24-HCO3-)=Na+-Cl--36
The results should be interpreted in the same way as it is done for the DG calculations: If the DG is significantly positive (>+6), a metabolic alkalosis is usually present because the rise in AG is more than the fall in HCO3-. Conversely, if the DG is significantly negative (<-6), then a hyperchloremic acidosis is usually present because the rise in AG is less than the fall in HCO3-.5 The Modified DG Equation is a very convenient and a quick way to estimate the mixed metabolic acid-base disorders as well as the dynamics of their development (by serial calculations). However, it can only be used if the clinical and other evidence of the underlying AG metabolic acidosis is still present. In our case DG after cardiac arrest was +12. This represents AG metabolic acidosis with metabolic alkalosis. Before the cardiac arrest DG was +4 representing solely AG metabolic acidosis. All this provides us with the conclusion that between the two tests the patient developed metabolic alkalosis, which was explainable by other clinical data (mainly by using the sodium bicarbonate during the ACLS measures).
References
1. Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007 Jan;2(1):162-174.
2. Haber RJ. A practical approach to acid-base disorders. West J Med 1991 Aug;155(2):146-151.
3. Rastegar A. Use of the DeltaAG/DeltaHCO3- ratio in the diagnosis of mixed acid-base disorders. J Am Soc Nephrol 2007 Sep;18(9):2429-2431.
4. Halperin ML, Kamel KS. Some observations on the clinical approach to metabolic acidosis. J Am Soc Nephrol 2010 Jun;21(6):894-897.
5. Wrenn K. The delta (delta) gap: an approach to mixed acid-base disorders. Ann Emerg Med 1990 Nov;19(11):1310-1313.
|