|
Abstract
Objective: Neonatal pneumothoraces are associated with high mortality. Prompt recognition to minimize its complications is paramount for ultimate outcome of these babies.
Methods: A retrospective case series study was carried out at Aga khan University Hospital, from January 2010 to December 2010 to determine the etiology and outcome of neonates with pneumothorax in a neonatal tertiary care unit.
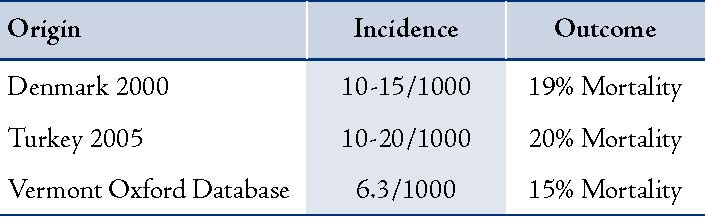
Results: Ten neonates diagnosed radiologically with pneumothoraces were included. M: F ratio was 1:2.3. Birth weight ranged from 1750-3600 grams with a mean of 2100 grams. The occurrence of pneumothoraces was 50% on the left side, 20% on right, and 30% were bilateral. Primary etiology included pneumonia and sepsis (30%), hyaline membrane disease (20%), meconium aspiration syndrome (20%) and congenital diaphragmatic hernia (10%). Spontaneous pneumothoraces were present in 20% of cases. In our study, the incidence of neonatal pneumothoraces was 2.5/1000 births compared to 10-15/1000 in Denmark, 10-20/1000 in Turkey and 6.3/1000 from Vermont Oxford Group. Despite the small number of cases, one incidental finding was the occurrence of pneumothorax, which declined in elective cesarean section after 37 weeks gestation i.e., 1.3 of 1000 births. Mortality was 60% determined mainly by the primary etiology and other co-morbid conditions.
Conclusion: The study showed a higher number of mortality cases (60%). Although, it was difficult to draw a conclusion from the limited number of cases, there may be a benefit on neonatal respiratory outcome to be obtained by better selection of mothers and by waiting until 37 weeks before performing elective cesarean section. Adequate clinician training in soft ventilation strategies will reduce the occurrence of pneumothoraces.
Keywords: Neonates; Pneumothoraces; Pneumothorax.
Introduction
Pneumothorax is the dissection of air into the pleural space; thus sufficient accumulation of air causes tension pneumothorax.1 Although sometimes asymptomatic, pneumothorax causes acute deterioration of a neonate in a neonatal intensive care.2 Prompt recognition and treatment of this condition is lifesaving. Pneumothorax typically causes worsening of tachypnea, grunting and cyanosis, as well as decreased breath sounds on the affected side. Detection of pneumothorax in a neonate depends on high index of suspicion and knowledge of its predisposing factors in different gestational age groups.
Methods
A retrospective case series study was carried out at Aga khan University Hospital, from January 2010 to December 2010 to determine the etiology and outcome of neonates with pneumothoraces. Neonates with no radiological confirmation were excluded. A pre designed performa was filled with different variables containing gender, birth weight, gestational age, primary etiology, signs and symptoms, mode of delivery, mean duration of illness, management and outcome. Categorical variables were identified and reported in percentages. Data was entered and analyzed using SPSS 17.0.
Results
The study group comprised of 10 neonates with male to female ratio of 1:2.3. Birth weight ranged from 1750 - 3600 grams with a mean of 2100 grams. Also, five were term, 4 preterm and 1 post-term. The onset was within the first week of life in all. Left sided (50%) pneumothoraces were more frequent than the right sided (20%) while 30% were bilateral. Signs and symptoms were tachypnea (46%), cyanosis (21%), irritability (13%), chest retraction (8%) and apnea (8%). The treatments performed were oxygen therapy alone in 2 babies and closed thoracotomy with underwater seal drainage needed in eight babies. One required thoracocentesis before closed thoracotomy. The Mean duration of air leakage was 9.7 hours, and the mean drainage time was 1.3 days. Primary etiology included pneumonia and sepsis (30%), hyaline membrane disease (20%), (out of which half receive no surfactant therapy for not achieving British Association of Perinatal Medicine [BAPM] guidelines), meconium aspiration syndrome (20%), and congenital diaphragmatic hernia (10%). Spontaneous pneumothoraces were found in 20% of cases (Table 1). The mean age in hours at diagnosis was 96 hours for the ventilated preterm (68 hours) and term (105.5 hours). Also, 70% required positive pressure ventilation post pneumothorax. Five babies (50%) were on ventilators when pneumothorax occurred.
There was an inadvertent high peak inspiratory pressure relative to gestational age and primary etiology noticed in these babies. There was recurrence in one baby and evidence of other air leaks (pneumomediastinum) in another. In a significant 40%, the diagnosis was radiological and not clinically suspected. In the current study, the incidence of neonatal pneumothoraces was 2.5/1000 births compared to 10-15/1000 in Denmark, 10-20/1000 in Turkey and 6.3/1000 from Vermont Oxford Group (Table 2). Neonates delivered by elective cesarean section showed a higher incidence of pneumothorax (2.9 of 1000 births) than did infants delivered by emergency cesarean section (1.53 of 1000 births) or by vaginal delivery (0.39 of 1000 births). This incidence was declined from 2.9 to 1.3 of 1000 births, if elective cesarean section was performed after 37 weeks of gestation. Mortality was 60%, determined mainly by the primary etiology and other co-morbid conditions.
Table 1: Causes of Pneumothoraces.

Table 2: Incidence of Pneumothoraces.
Discussion
Intensive care treatment modalities have become increasingly dependent on positive-pressure ventilation, central venous catheter placement and other causes that potentially induce iatrogenic pneumothorax.3 Most small pneumothoraces resolve spontaneously, but larger and tension pneumothoraces require evacuation of the air in the pleural cavity.4,5 Neonatal Pneumothoraces is a major cause of acquired displacement of the heart (and of the mediastinum). It is relatively common in preterm infants, especially when they are ventilated, although recent advances in ventilatory management of these tiny babies seem to have reduced its incidence.6 Moreover, it is relatively common among neonates who have a variety of lung diseases, such as meconium aspiration, lung hypoplasia, disorders of reduced amniotic fluid volume (renal agenesis), or decreased breathing movements (neuromuscular diseases, oligohydramnios).7 Predisposing conditions are found in other infants, such as congenital emphysema and cystic malformations, or pneumothoraces may be the consequence of bronchial compression in patients who have the syndrome of pulmonary valve agenesis (in whom the dilated pulmonary arteries compress the adjacent bronchi, trapping the air distally through a valve mechanism). Familial cases have been reported in whom an intrinsic "weakness" of the lung tissue has been suggested.8
Spontaneous Pneumothoraces is relatively uncommon, although it has been estimated that 1% to 2% of healthy neonates may be affected by clinically silent Pneumothoraces.9 Symptomatic Pneumothoraces, which is even less uncommon, is characterized by respiratory distress that may vary from a simple increase in respiratory rate to severe tachypnea, dyspnea and cyanosis. The onset may be sudden or subtle, and asymmetric chest movement may be evident. There may be absence of breath sounds on the affected side, and the heart sounds can be displaced to the opposite side.
The treatment of neonatal Pneumothoraces depends on the clinical picture. Small, asymptomatic Pneumothoraces (occupying <15% of hemi-thorax volume) may be left untreated, waiting for spontaneous reabsorption of air; in symptomatic cases with cardiorespiratory involvement, drainage is mandatory. In tension pneumothorax, a butterfly needle and syringe can be used to temporarily evacuate free air from the pleural space. This procedure is usually performed at the bedside. Definitive treatment is insertion of an 8 or 10 French chest tube attached for continuous suction.10 Follow-up auscultation, transillumination and X-ray will confirm that the tube is functioning properly.
Conclusion
In 2010, we experienced 10 cases of neonatal pneumothorax in the neonatal intensive care unit, Aga Khan University Hospital. The incidence of neonatal pneumothorax was 2.5/1000 births and there were 2 spontaneous pneumothoraces and 8 secondary pneumothoraces. The overall hospital mortality was 60% would be an over estimate probably because of limited number of cases. The rate of complication was 46% in our study. The complications were metabolic acidosis, atelectasis, pleural effusion, pulmonary hemorrhage and pneumonia.
There may be a benefit on neonatal respiratory outcome to be obtained by better selection of mothers and by waiting until week 37 weeks before performing elective cesarean section. Although, it is difficult to draw a conclusion from these limited number of cases. The authors also highlighted the importance of adequate clinician training in "gentle" bag-and-mask ventilation and soft ventilation strategies in their local setting considering the statistically high rate (50%) of pneumothoraces in ventilated babies. The study did not elucidate whether inadvertent over ventilation (indicated by low carbon dioxide tension) caused the pneumothorax or increased clinical interventions secondary to primary etiology and other comorbids, including reintubation were actually associated with these pneumothoraces.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Leigh-Smith S, Davies G. Tension pneumothorax: eyes may be more diagnostic than ears. Emerg Med J 2003 Sep;20(5):495-496.
2. Boo NY, Cheah IG; Malaysian National Neonatal Registry. Risk factors associated with pneumothorax in Malaysian neonatal intensive care units. J Paediatr Child Health 2011 Apr;47(4):183-190.
3. Singh SA, Amin H. Familial spontaneous pneumothorax in neonates. Indian J Pediatr 2005 May;72(5):445-447.
4. Ainsworth AP, Ruager AR, Holtved E. [Neonatal pneumothorax]. Ugeskr Laeger 2000 Dec;162(49):6679-6682.
5. Esme H, Doğru O, Eren S, Korkmaz M, Solak O. The factors affecting persistent pneumothorax and mortality in neonatal pneumothorax. Turk J Pediatr 2008 May-Jun;50(3):242-246.
6. Holloway VJ, Harris JK. Spontaneous pneumothorax: is it under tension? J Accid Emerg Med 2000 May;17(3):222-223.
7. Carey B. Neonatal air leaks: pneumothorax, pneumomediastinum, pulmonary interstitial emphysema, pneumopericardium. Neonatal Netw 1999 Dec;18(8):81-84.
8. Kugelman A, Riskin A, Weinger-Abend M, Bader D. Familial neonatal pneumothorax associated with transient tachypnea of the newborn. Pediatr Pulmonol 2003 Jul;36(1):69-72.
9. Hansen TG, Jepsen SB, Schierbeck J, Andersen PK. [Neonatal pneumothorax]. Ugeskr Laeger 2001 Feb;163(7):936-937.
10. Ferrie EP, Collum N, McGovern S. The right place in the right space? Awareness of site for needle thoracocentesis. Emerg Med J 2005 Nov;22(11):788-789.
|