|
Abstract
Objectives: This is a report on the types and patterns of inborn errors of metabolism (IEMs) of amino acids, organic acids and fatty acids oxidation detected by Tandem Mass Spectrometry for a period of 10 years (1998-2008) at Sultan Qaboos University Hospital (SQUH), the major centre for diagnosis and management of IEM in Oman.
Methods: Tandem mass spectrometry (MS/MS) was used in the initial screening and diagnosis of IEMs in high risk neonatal and pediatric populations.
Results: Out of 1100 patients investigated, 119 were detected positive for IEM by MS/MS spectrometry. Twenty six different metabolic diseases were detected. Patients were categorized into three major groups: a) 54 with amino acids and urea cycle disorders, b) 35 with organic acid disorders, and c) 30 with fatty acid oxidation disorders. The commonest conditions encountered were maple syrup urine disease (MSUD), phenylketonuria (PKU), propionic and isovaleric acidurias, as well as HMG-CoA lyase deficiency and glutaric aciduria type II (GA-II). Most of these IEMs were over representedin babies born to consanguineous parents, which is consistent with the recessive autosomal inheritance.
Conclusion: This study shows that various types of IEMs, reported elsewhere, were also prevalent in Oman, but the pattern of prevalence and distribution is different. The situation, therefore, warrants the development of a nationwide screening and prevention program.
Keywords: Oman; Mass spectrometry; Inborn errors.
Abbreviations: ASA: Arigininosuccinic aciduria, BKT: β-ketothiolase deficiency, BPD-PKU: Biopterin-dependent phenylketonuria, CPT-I: Carnitine palmitoyl transeferase-I, EMA: Ethylmalonic aciduria, GA-I: Glutaric aciduria type-I, GA-II: Glutaric aciduria type-II, HMG-CoA Lyase: 3-hydroxy-3-methylglutaryl-CoA lyase deficiency, LCHAD: Long-chain hydroxyl acyl CoA dehydrogenase deficiency, MCD: Multiple carboxylase deficiency, MCAD: Medium-chain acyl CoA dehydrogenase deficiency, MGA: 3-methylglutaconic aciduria, MMA: Methylmalonic acidemia, MSUD: Maple syrup urine disease, NKHG: Non-ketotic hyperglycinemia, OTC: Ornithine transcarbamylase, PGA: Pyroglutamic aciduria, PKU: Classical Phenylketonuria, PPA: Propionic acidemia, SCHAD: Short-chain hydroxyacyl CoA dehydrogenase deficiency, VLCAD: Very long-chain acyl CoA dehydrogenase deficiency.
Introduction
Tandem mass spectrometry (often abbreviated as tandem MS or MS/MS) is considered an important new technology for neonatal screening and diagnosis of inborn errors of metabolism (IEMs). It permits the detection and quantification of many IEMs in a single blood spot for most amino acids, urea cycle disorders, organic acids and fatty acid oxidation defects. Furthermore, MS/MS has introduced the concept of multiple metabolite analyses for the detection of numerous metabolic disorders in a single analytical run.1-3 At present, MS/MS is being used for screening and diagnosis of IEMs in newborns and sick infants in many clinical biochemical laboratories in the USA, Europe, Australia, Japan, and other countries.4-11
SQUH is the main referral hospital for IEMs in Oman. An organized diagnostic service for IEMs for high-risk babies was established at SQUH in 1998. During 1998-2002, samples were tested at King Faisal Specialist Hospital, Saudi Arabia. In 2002, MS/MS spectrometry was introduced at SQUH. Here, the types and patterns of IEMs encountered at SQUH over a period of 10 years (1998-2008) were reviewed. This represents only part of the disease load in a total Omani population of 1.6 million people. Clinical descriptions of many of these patients were previously reported.12-13
Methods
High-risk babies or children from regional hospitals are routinely referred to the Metabolic Division of Child Health Department at SQUH. A total of 1100 patients were referred and investigated for inborn errors of metabolism during a period of 10 years (1998-2008). Whole blood samples were drawn by heel prick or venipuncture from high-risk babies and spotted on Guthrie filter cards No. 903 Whatman paper (Schleicher and Schuell, Dassel, Germany). Four spots of whole blood were collected and allowed to dry at room temperature for at least three hours. Samples were then sent to the laboratory in plastic envelopes and stored at 4°C until analyzed.
In an attempt to reveal the pattern of distribution of IEMs in Oman, the geographical location as well as the ethnic and tribal origin of parents was examined. The Omani population was divided into three ethnic groups: Arabs of the north, Arabs of the south (Dhofari), and Omanis of Asian descent. Northern Arabs included all Arab tribes occupying the northern parts of Oman. Southern Arabs included all the Arabs from Dhofar and southern parts of Oman. Omanis of Asian Origin included migrants from Iran, India, Pakistan and Afghanistan.
Mass spectrometry is a technique used for identifying and quantifying analytes based on their molecular mass and charge. For analysis by a mass spectrometer, the analyte of interest must be converted into ions. Ions are separated by electromagnetic fields, detected by a quantitative method and processed into mass spectra (Fig. 1) according to their mass to charge ratio (m/z). Therefore, the main four components of MS instrument are sample inlet, ion source, mass analyzer, and a detector. The vertical axis represents ion intensity expressed as a % of the largest peak in the spectrum. Internal standards are represented with a small superscript star.
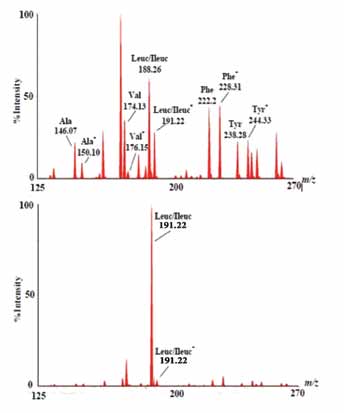
Figure 1: MS/MM spectra of amino acids butyl esters using an NL 102 scan. Top Panel: Normal newborn profile. Bottom panel: Profile for newborn with Maple Syrup Urine Disease (MSUD). The horizontal axis represents mass to charge ratio (m/z).
In Electrospray-ionization tandem mass spectrometry (ESI-MS/MS), a sample is first dissolved in a mixture of water and organic solvent (e.g., acetonatrile). Then, the extract is derivatized with butanol to form butyl esters of amino acids, organic acids, and fatty acids. As the mass spectrometer cannot detect uncharged molecule, the analyte must be ionized before it is introduced to the first MS (MS 1). Ionization of the derivatized analyte is achieved by electrospray ionization in which the sample effluent is passed through a small capillary to which a high voltage has been applied. The ionization process involves the transfer of a charge to the solvent droplets, evaporation of the solvent, and finally, production of positively and negatively charged ions. This vaporized and ionized mixture enters the MS 1, which functions as a separating device and allows only the ion(s) of interest to pass through. Ions passing through the MS 1 are called precursor or parent ions. Once the ion passes through the MS 1, it enters the collision cell where fragmentation takes place.
Fragmentation is typically achieved by introduction of an inert gas such as argon into the collision cell. The fragments generated in the collision cell are called product or daughter ions. In the second mass spectrometer (MS2), the product or daughter ions are analyzed in the same manner as in the first mass spectrometer. Products or daughter ions in the MS2 can be correlated with the parents ions produced in the MS 1. This process enables unique mass spectrometry such as precursor ion scans and neutral loss scans. Since certain compound classes share common fragment ions of neutral fragment molecules, a special analysis can be set up to only detect precursor ions with a particular fragment. This results in an ability to measure only a particular chemical class or subset of molecules without detecting the hundreds of molecules that are not of interest.
ESI-MS/MS was carried out on a Quattro Micro (Manchester, UK), triple quadrupole spectrometer, equipped with a Waters Alliance 2790 HPLC pump, with minor modification of methods developed by Chance et al.1‑2 and Rashed et al.3
A 3 mm disk from a selected spot from the Guthrie card was punched out using a standard hole punch in each of a 96-well polypropylene microtitre plate. The sample in the blood spot punch in each well was then extracted by adding 100 μl of isotope-labeled internal standard solution of amino acids and acylcarnitines (Cambridge Isotope Labs, Andover, MA, USA) and incubated at room temperature for 30 minutes. The extracted sample was then transferred to another plate. The solvent was gently evaporated and 60 μl of butanolic HCl was added. The plate was sealed with special heat-resistant tape and heated at 65oC for 15 minutes. The solvent was evaporated again and the residue was finally reconstituted in 100 μl of the mobile phase (acetonatrile/water, 80/20 v/v) and fed into the system through the HPLC pump.
Neolynx software (Neolynx Inc., Glendale, California, USA) was used for processing MS/MS data. Neolynx operates by calculating the ratio of the intensity of the mass spectral peak for the analyte to the intensity of the mass spectral peak for a pre-defined internal standard. If the concentration of the internal standard is known then the concentration of the analyte may be calculated by multiplying the peak intensity ratio by the concentration of the internal standard. Samples were analyzed in duplicates in the same analytical run. If a sample is flagged as abnormal, a repeat sample for MS/MS analysis was requested. If the second sample agreed with the first sample result, the diagnosis was then confirmed by urine organic acids analysis, plasma amino acids analysis, or direct enzyme assay; all analyzed by Biomnis laboratory, France (BIOMNIS Specialised Medical Pathology, International Division, Lyon Cedex 7 – FRANCE).
Results
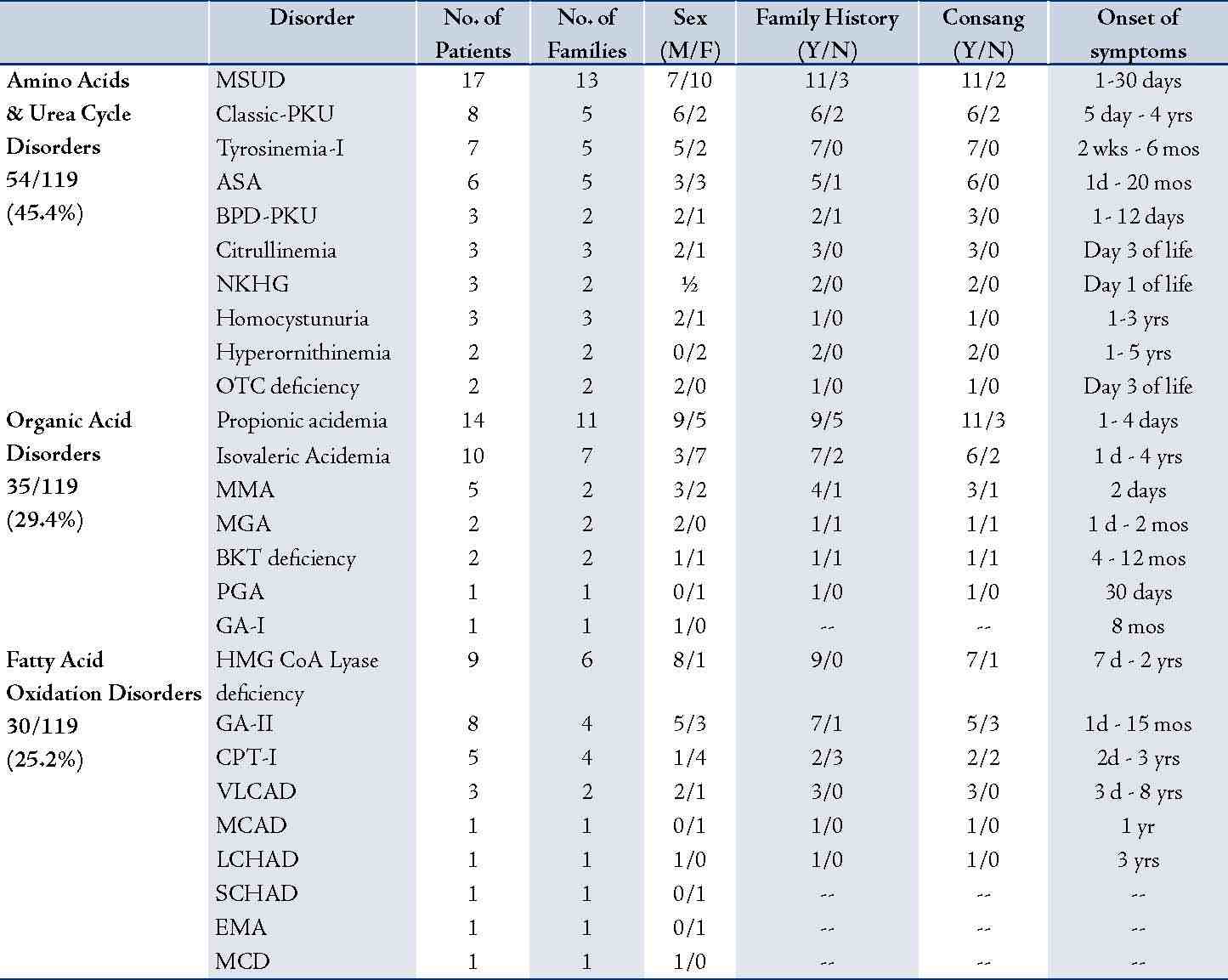
During the period of May 1998 - July 2008, a total of 1100 patients were tested, wherein 119 were positive for an IEM. The important characteristics of the 119 patients with diagnosed IEM are shown in Table 1. The 119 patients suffered from three main categories of IEM, namely; amino acids and urea cycle defects, organic acid disorders, and fatty acid oxidation disorders. The 119 patients belonged to 89 different families; as some families had more than one affected child with the same disorder. Fifty six percent (67/119) of the patients were males and 44% (52/119) were females. Information regarding family history of the same illness or unexplained death was obtained in 77 patients while consanguinity was confirmed in 73 patients. It was also observed that symptoms of IEMs generally presented as early as one day or could be delayed for up to eight years of life. Most of the patients (86%) were Northern Arabs while 12% were of Asian origin. Arabs of the south comprised only 2% of the studied population. Furthermore, it was noted that 47% (48/102) of the Northern Arabs and 86% (12/14) of the Omanis of Asian origin came from the north coastal region of Oman.
Some specific diseases were confined to Arabs from Northern Oman such as MSUD (17/17), BPD-PKU (3/3), some urea cycle defects [ASA (4/6) hyperornithinemia (2/2), Citrullinemia (3/3), OTC deficiency (2/2)], VLCAD (3/3), HMG-CoA lyase deficiency (9/9), CPT-I deficiency (5/5), and 3-Methylglutaconic aciduria (2/2). Arabs of the North also had a large portion of PPA (11/14), IVA (8/10), tyrosinemia type-1 (6/7), classical homocystinuria (2/3), and GA-II (4/8).
Table 1: Characteristics of the 119 Omani neonates, babies and children with inborn errors of metabolism diagnosed at Sultan Qaboos University Hospital during 1998-2008 using tandem mass spectrometry.
Discussion
In this retrospective study, 119 patients were diagnosed with IEMs out of 1100 high-risk Omani neonates, babies and children during the period of May 1998 to December 2008. Clinical diagnosis was initially confirmed by a rapid screen using MS/MS spectrometry. The initial pick up rate (1 out of 9.2 tests) for MS/MS recorded here is, therefore, fairly high. The definitive diagnosis was made by plasma amino acid analysis, urine organic acid analysis or direct enzyme assays.
The number of IEM patients from each of the five regions of Oman seems to be more or less proportional to the percentage of the population in each region; except, Dhofar region, where the number of IEM patients is lower than expected. This is a direct reflection of the small number of patients referred from Dhofar; a geographically distant region of Oman.
In this study, IEMs are over-represented in babies born to consanguineous parents and most patients were found to have a positive family history of an IEM or of unexplained death among their families. These findings are consistent with the recessive autosomal inheritance of IEMs and reflect the significant contribution of consanguinity in Oman in this particular health problem.14 It was also noticed that many families have more than one affected child with IEM, which can be attributed to the lack of early detection of such disorders, as well as the lack of parental genetic counseling. Ignorance about IEMs and the lack of specialized healthcare centers and practitioners in Oman must have further contributed to this health problem.
The initial symptoms of IEMs may appear as early as day one of life. In this study, the onset of symptoms ranged from 1 day to 8 years. Early detection and treatment of individuals at risk of IEMs before the onset of symptoms can prevent or at least, reduce serious neurological and developmental damage.15
Conclusion
This retrospective review of IEMs at SQUH shows that different varieties of IEMs reported elsewhere were also prevalent in Omani communities and therefore, warrants the development of a nationwide screening program, as well as a systematic cost-effective prevention program,16 similar to those already in place in the Arabian Gulf countries.10,17
Acknowledgements
The authors reported no conflict of interest for this work. SAR was a recipient of SQU scholarship for MSc degree. We are grateful for Mrs. Taruna Dutt for typing the manuscript.
References
1. Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem 1995;41:62-68.
2. Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry of multianalyte screening of dried blood specimens from newborns. Clin Chem 2003;49:1797-1817.
3. Rashed MS, Ozand PT, Bucknall MP, Little D. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res 1995;38:324-331.
4. Ziadeh R, Hoffman EP, Finegold DN, Hoop RC, Brackett JC, Strauss AW et al. Medium chan acyl-CoA dehydrogenase deficiency in Pennsylvania: neonatal screening shows high incidence and unexpected mutation frequencies. Pediatr Res 1995;37:675-678.
5. Shigematsu Y, Hirano S, Hata I, Tanaka Y, Sudo M, Sakura N et al. Newborn mass screening and selective screening using electrospray tandem mass spectrometry in Japan. J Chromatogr B Analyt Technol Biomed Life Sci 2002:776:39-48.
6. Schulze A, Linder M, Kolmuller D, Olgemoller K, Matatepek E, Hoffmann GF. Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 2003;111:1399-1406.
7. Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 2003;348:2304–2312.
8. Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S. Clinical effectiveness and cost effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess 2004;8:1-121.
9. Borrajo GJ. Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis 2007;30:466-481.
10. Saddallah AA, Rashed MS. Newborn screening: experiences in the Middle East and North Africa. J Inherit Metab Dis 2007;30:482-489.
11. Therrel BL, Adams J. Newborn screening in North America. J Inherit Metab Dis 2007;30:447-465.
12. Joshi SN, Hashim J, Venugopalan P. Pattern of inborn errors of metabolism in an Omani population of the Arabian Peninsula. Ann Trop Paediatr 2002;22:93-96.
13. Joshi SN, Venugopalan P. Clinical characteristics of neonates with inborn errors of metabolism detected by Tandem MS analysis in Oman. Brain Dev 2007;29:543-546.
14. Rajab A, Patton MA. A study of consanguinity in the Sultanate of Oman. Ann Hum Biol 2000;27:321-326.
15. American College of Medical Genetics. Report on Newborn screening: toward a uniform screening panel and system. Genet Med 2006;8 Suppl 1:1S-252s.
16. Pollitt RJ, Green A, McCabe CJ Booth A, Cooper NJ, Leonard JV et al. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technol Assess 1997;1:1-191.
17. Al-Hosani H, Salah M, Saade D, Osman H, AlZahid J. United Arab Emirates National Screening Program: an evaluation 1998-2000. East Mediterr Health J 2003;9:324-332.
|