Cupping has been used in both Eastern and Western countries through the ages. For example, bull-horn cupping has been practiced in China since the second century, and artifacts demonstrate its use in ancient Greece and Rome.1 The two most common cupping practices are dry and wet cupping. Dry cupping is frequently practiced in countries in the Far East, whereas wet cupping is more favored in the Middle East and Eastern Europe.2 Cupping is also known as Al-Hijamah in Arabic, meaning expansion, sucking, and bloodletting to return the body to its natural condition.3
According to Avicenna, a prominent Persian physician, wet cupping facilitates blood purification or clearance, especially for the skin and its adjacent organs.3 This assertion is further expanded in blood detoxification theory,4 which states that the practice of wet cupping causes increased blood flow to the affected area. It induces blood purification or toxin clearance and improves the local nutrition state. The increase in blood flow to the affected area later will boost the body’s metabolism and eliminates pathogenic factors.
In evidence-based medicine, wet cupping therapy is indicated for pain relief and the reduction of inflammation caused by, for example, musculoskeletal pain.2,5–8 It is also recommended to improve blood flow,9 to remove oxidants and oxidative stress,10 and even to treat infertility.11 Earlier studies have investigated the physiological mechanisms of wet cupping that induce health benefits. Emerich et al,12 assessed the effects of cupping in 12 volunteers and found that levels of lactate, pyruvate, glucose, and glycerin increased significantly in the surrounding tissues, suggesting anaerobe metabolism in the subcutaneous tissues near the cupping site. Wet cupping was also found to reduce diastolic blood pressure and fasting blood sugar (FBS) in a study involving 16 healthy participants that assessed the short-term effects of cupping on blood pressure and FBS within 30 minutes and 48 hours, respectively.13 However, the study found no significant improvement in systolic blood pressure (SBP).
The Malaysian Ministry of Health acknowledges the use of traditional and complementary medicine (TCM) to treat disease and maintain health. In 2000, this ministry established the Herbal Medicine Research Centre. The Global Information Hub on Integrated Medicine launched a year later, followed by the formation of the Traditional and Complementary Division in 2004. Since, guidelines have been developed, including ‘Guidelines for Traditional & Complementary Medicine Practice: Cupping’ in 2013, indicating the successful integration of TCM into modern medicine. However, cupping is still not included among the seven modalities of TCM practices offered by the TCM units at Malaysian government hospitals.14
The increasing prevalence of metabolic syndrome in Malaysia heightens the need for promising and cost-effective preventive measures to curb this condition. While the prevalence of diabetes mellitus in Malaysia is 22.9%15, the reported prevalence of metabolic syndrome is 27.5%.16 There is insufficient evidence regarding the effectiveness of cupping for metabolic syndrome or cardiovascular risk prevention because few trials have assessed repeated outcomes. A clinical trial with a sufficient number of respondents and specific blood parameters measured at repeated intervals is warranted to fill this gap in scientific understanding. Therefore, our study aimed to evaluate the effects of wet cupping on FBS, renal function parameters, and endothelial function in healthy individuals. Our study thus provides preliminary support for the therapeutic outcomes of practicing wet cupping on the human body.
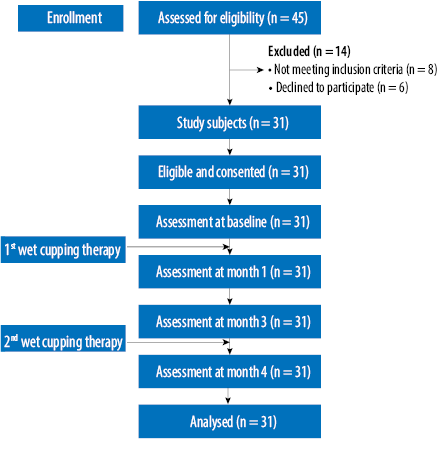
Figure 1: Flowchart of recruitment and study conditions.
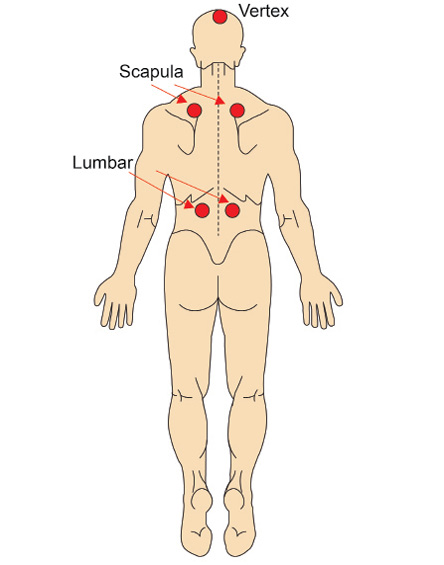
Figure 2: Five-point wet cupping site.
Methods
We conducted a single-arm intervention study to evaluate the effects of wet cupping therapy on healthy individuals. The study was conducted from January to June 2012. We enrolled participants through advertisements in a local email group and telephone calls to university staff. Sample size calculations were based on repeated measures using STATA software. The required sample size was 31 based on the mean blood glucose parameter before the intervention (6.30±3.0 mmol/L)17 and a 20% drop-out rate. Individuals aged 30–60 years without a chronic disease, blood disorder, or history of dyslipidemia were eligible for inclusion. Figure 1 illustrates the participants’ recruitment patterns. The study protocol was approved by the Human Research Ethics Committee, Universiti Sains Malaysia (reference no. USMKK/PPP/JEPeM [243.3.(13)]). Signed informed consent was obtained from each participant before the start of the study.
Cupping was performed at the Clinical Public Health Laboratory, Universiti Sains Malaysia, by trained researchers with serial training from qualified cupping therapy personnel who were registered with the Traditional and Complementary Alternative Division, Ministry of Health, Malaysia. The participants received wet cupping therapy two times: at the start of the study and three months later. The researchers used 5 cm and 1.5 cm disposable plastic cups as well as disposable lancet needles for safety purposes. Five treatment points were located: both scapulas, both lumbar regions, and the vertex [Figure 2]. Before the procedure, the participants were asked to lie topless on a couch. Each wet cupping treatment procedure lasted approximately 20 minutes. The wet cupping procedure was applied according to the pre-defined aseptic technique18,19 delineated in Box 1.
Box 1: Aseptic wet cupping technique.
Demographic information was obtained at baseline. SBP and three types of blood analyses were also measured: FBS, renal function parameters (urea, creatinine, and uric acid), and von Willebrand factor (vWF). Ten millimeters of venous blood was taken from each participant following eight hours of overnight fasting; 4 mL of venous blood was inserted into a plain tube for renal function parameters and 2 mL into a tube with oxalate/fluoride for FBS testing along with another 4 mL for the vWF ELISA test using an EDTA tube. The blood was transported within four hours to an accredited laboratory for analysis. The measurements were repeated at one, three, and four months of the study. However, the vWF test was only repeated at four months.
Statistical analyses were performed using SPSS version 19. The baseline characteristics were presented descriptively, while repeated-measures analysis of variance (ANOVA) was performed for the repeated measures of the same variables. The mean comparisons of the outcome measurements were assessed at baseline, and months one, three, and four. p-values < 0.050 were considered significant.
Table 1: Respondents’ characteristics and health outcome measurements at baseline (n = 31).
|
Age, years |
44.9 ± 6.4 |
|
Gender, female/male |
14 ± 45.2/17 ± 54.8* |
|
Race, Malay/non-Malay |
31 ± 100/0 ± 0.0* |
|
Fasting blood sugar, mmol/L |
5.0 ± 0.4 |
|
Serum urea, mmol/L |
4.2 ± 0.7 |
|
Serum creatinine, μmol/L |
70.2 ± 13.2 |
|
Serum uric acid, μmol/L |
371.8 ± 96.2 |
|
Systolic blood pressure, mmHg |
127.2 ± 12.6 |
*frequency (percentage).
SD: standard deviation.
Results
Out of the 45 healthy adults screened, eight did not fulfill the study protocols, and another six refused to participate [Figure 1]. Table 1 illustrates the descriptive analysis of respondents and the health benefits measurement at baseline. The mean age of respondents was 44.9 years, with slightly more males than females, and all the respondents were Malay. All the measured health outcome parameters were within the normal range. Throughout the study, no adverse events were noted like hematoma, bleeding, hypotension, or significant skin reactions.
A repeated measure of ANOVA within-group analysis was tested for underlying assumptions; normality of residuals, homogeneity of variance, and compound symmetry. Mauchly’s test of sphericity showed the assumption of compound symmetry were violated for serum uric acid (p = 0.005), serum creatinine (p = 0.007), vWF (p < 0.001), and SBP (p = 0.003) that required the correction of a degree of freedom using Huynh-Feldt estimates of sphericity. The univariate test for comparison within-group differences revealed significant differences in the mean of all health outcome parameters. Thus, pairwise comparison with confidence interval (CI) adjustment (Bonferroni) was conducted for the significant variables [Tables 2 and 3]. Results show FBS was significantly lower at one month (4.8±0.4), three months (4.8±0.4), and four months (4.7±0.5) compared to baseline (5.0±0.4). There was also a significant reduction of serum urea at one month (3.8±0.8), three months (3.8±0.6), and four months (3.6±0.6) compared to baseline (4.2±0.7). Similarly, serum creatinine following wet cupping therapy showed a significant reduction at one month (63.1±11.0), three months (66.1±14.7), and four months (63.3±11.9) compared to baseline (70.2±13.2). For the serum uric acid, there was a reduction at one month (322.8±82.7), three months (338.5±88.7), and four months (326.3±87.1). However, a significant difference compared to baseline was found only at one and four months. Mean SBP was reduced throughout the measurements; however, significant reductions were found at one and four months with the respective mean difference of 5.4 and 6.9 compared to baseline. Finally, the vWF also showed a significant reduction at four months (105.6±13.9) compared to baseline (111.0±18.0), with a mean difference of 5.3. Overall findings indicate that wet cupping therapy significantly reduced serum FBS by 3.7–6.7%, renal functions by 8.1–13.2%, SBP by 4.2–5.5%, and vWF by 4.8%.
Table 2: Pairwise comparison of fasting blood sugar, renal function parameters, and endothelial function following wet cupping therapy based on time (n = 31).
|
Fasting blood sugar, mmol/L |
|
|
|
Baseline – one month |
0.1 (0.01, 0.36) |
0.033 |
|
Baseline – three months |
0.2 (0.06, 0.41) |
0.004 |
|
Baseline – four months |
0.3 (0.10, 0.59) |
0.003 |
|
Serum urea, mmol/L |
|
|
|
Baseline – one month |
0.3 (0.08, 0.69) |
0.007 |
|
Baseline – three months |
0.3 (0.03, 0.65) |
0.030 |
|
Baseline – four months |
0.5 (0.10, 0.94) |
0.009 |
|
Serum creatinine, μmol/L |
|
|
|
Baseline – one month |
7.1 (3.42, 10.84) |
< 0.001 |
|
Baseline – three months |
4.0 (0.83, 7.30) |
0.008 |
|
Baseline – four months |
6.8 (2.92, 10.82) |
< 0.001 |
|
Serum uric acid, μmol/L |
|
|
|
Baseline – one month |
48.9 (11.84, 86.03) |
0.005 |
|
Baseline – three months |
33.2 (-5.40, 71.98) |
0.128 |
|
Baseline – four months |
45.4 (0.44, 90.40) |
0.047 |
|
Systolic blood pressure, mmHg |
|
|
|
Baseline – one month |
5.4 (0.42, 10.48) |
0.028 |
|
Baseline – three months |
5.3 (-0.17, 10.95) |
0.062 |
|
Baseline – four months |
6.9 (1.87, 12.00) |
0.003 |
|
von Willebrand factor, IU/dL |
|
|
*Repeated measure of ANOVA within-group analysis was applied, followed by pairwise comparison.
MD: mean difference; CI: confidence interval.
Table 3: Health outcomes changes (%) following wet cupping therapy (n = 31).
|
FBS, mmol/L |
5.0 ± 0.4 |
4.8 ± 0.4
(â 3.7)* |
4.8 ± 0.5
(â 4.5)* |
4.7 ± 0.5
(â 6.7)* |
|
Serum urea, mmol/L |
4.2 ± 0.7 |
3.8 ± 0.82
(â 9.3)* |
3.8 ± 0.6
(â 8.1)* |
3.68 ± 0.6
(â 12.4)* |
|
Serum creatinine, μmol/L |
70.2 ± 13.2 |
63.1 ± 11.0
(â 10.1)** |
66.1 ± 14.7
(â 5.8)* |
63.3 ± 11.9
(â 9.8)** |
|
Serum uric acid, μmol/L |
371.8 ± 96.2 |
322.8 ± 82.7
(â 13.2)* |
338.5 ± 88.7
(â 9.0) |
326.3 ± 87.1
(â 12.2)* |
|
SBP, mmHg |
127.2 ± 12.6 |
121.7 ± 13.5
(â 4.3)* |
121.8 ± 9.2
(â 4.2) |
120.2 ± 8.2
(â 5.5)* |
SD: standard deviation; FBS: fasting blood sugar; SBP: systolic blood pressure; vWF: von Willebrand factor; â: reduced.
*p < 0.050
**p < 0.001 using repeated measure of ANOVA within-group analysis.
Discussion
We investigated the effects of wet cupping therapy on biochemical and physiological body responses. The significant improvements in FBS, renal function parameters, SBP, and vWF indicated the substantial positive effects of wet cupping in improving the body’s physiological mechanisms. In a randomized, controlled trial of 60 healthy subjects, significant positive biochemical parameter changes were detected within 10 days of wet cupping.20 Notably, FBS, blood pressure, and renal function were substantially improved. The results of this study support and confirm the short-term health benefits of wet cupping therapy.
Urea and creatinine levels represent the glomerular filtration rate. A high level of urea can play a role in predicting the development of hypertension and mediating the systemic inflammatory response that is linked to cardiovascular events.21 Increasing serum creatinine levels indicate that the glomerular filtration rate has been compromised.22 Serum creatinine is used to estimate the glomerular filtration rate and classify the stage of chronic kidney disease to determine the required treatment options and modalities.23 Thus, reductions in these parameters due to wet cupping may have beneficial health impacts by maintaining body health and preventing metabolic diseases.
The vWF is a glycoprotein that is released into the circulation from endothelial cells following vascular injury. It carries coagulation factor VIII, osteoprotegerin, galectins, and several other proteins to mediate platelet adhesion and aggregation.24 vWF levels can be used as an indicator of cardiovascular events and stroke, especially in high-risk populations.25 Therefore, a reduction in vWF following wet cupping may imply improvements in liver and spleen function for the clearance of vWF or indicate improvements in endothelial cell function through reductions in thrombotic formation. Findings from previous studies have shown that the positive effects of cupping include improvements in blood flow, especially underneath affected areas.2,9 This further explains and supports the possible mechanism involved in helping the body’s physiological clearance when wet cupping is applied.
The lack of adverse events in this study further validates the safety of wet cupping therapy. In an overview of systematic reviews on the effectiveness and safety of cupping therapy, hematoma, increased pain, and tingling following cupping treatment were only reported in one of the eight reviews.6 Furthermore, Mahdavi et al,26 also reported no serious health effects following cupping treatment. Additionally, a review of 16 studies related to cupping found that adverse events were rare, and most of them were avoidable if performed by trained cupping personnel.1
A limitation in the interpretation of the results of this single-arm study design was the absence of a control group. Consequently, it was not possible to distinguish between the effects of cupping therapy, the placebo effect, and the effects of spontaneous improvement in the body’s physiology. Uncontrolled factors such as exercise, sufficient rest, a balanced diet, and other healthy lifestyle habits could be potential confounders behind the positive outcomes rather than cupping therapy. However, our study established the baseline parameter measurements to reduce the bias in the interpretation of the efficacy data. The participation of the volunteers in this study was subject to selection bias, which limits the extrapolation of its findings to the general healthy population. However, the promising positive findings concerning cupping therapy support the need and rationale for further investigation through the application of a more robust study design.
Conclusion
Wet cupping therapy has short-term health benefits that can lead to improvements in renal function and the prevention of metabolic disease. These potential effects of wet cupping suggest new evidence and perspectives on the reduction and prevention of cardiovascular risks. Its low costs, non-invasiveness, and minimal need for training suggest the possibility of promoting wet cupping as complementary medicine to benefit human health in the future. However, support for these preliminary findings is needed via further research that includes a control group to ascertain the long-term therapeutic health benefits of cupping therapy.
Disclosure
The authors declared no conflicts of interest. The study was funded by the Ministry of Higher Education Malaysia who provided an Exploratory Research Grant Scheme (grant no. 203/PPSP/6730025).
Acknowledgements
We would like to thank the Universiti Sains Malaysia for supporting this study.
references
- 1. Kim T-H, Kim KH, Choi J-Y, Lee MS. Adverse events related to cupping therapy in studies conducted in Korea: a systematic review. Eur J Integr Med 2014;6(4):434-440.
- 2. Ullah K, Younis A, Wali M. An investigation into the effect of cupping therapy as a treatment for anterior knee pain and its potential role in health promotion. Internet J Altern Med 2007;4(1):1-9.
- 3. Ghods R, Sayfouri N, Ayati MH. Anatomical features of the interscapular area where wet cupping therapy is done and its possible relation to acupuncture meridians. J Acupunct Meridian Stud 2016 Dec;9(6):290-296.
- 4. Al-Bedah AM, Elsubai IS, Qureshi NA, Aboushanab TS, Ali GI, El-Olemy AT, et al. The medical perspective of cupping therapy: effects and mechanisms of action. J Tradit Complement Med 2018 Apr;9(2):90-97.
- 5. Arslan M, Gökgöz N, Dane Ş. The effect of traditional wet cupping on shoulder pain and neck pain: a pilot study. Complement Ther Clin Pract 2016 May;23:30-33.
- 6. Cao H, Han M, Zhu X, Liu J. An overview of systematic reviews of clinical evidence for cupping therapy. Journal of Traditional Chinese Medical Sciences 2015;2(1):3-10.
- 7. Farhadi K, Schwebel DC, Saeb M, Choubsaz M, Mohammadi R, Ahmadi A. The effectiveness of wet-cupping for nonspecific low back pain in Iran: a randomized controlled trial. Complement Ther Med 2009 Jan;17(1):9-15.
- 8. Lee MS, Kim J-I, Ernst E. Is cupping an effective treatment? An overview of systematic reviews. J Acupunct Meridian Stud 2011 Mar;4(1):1-4.
- 9. Wei LI, Piao SA, Meng XW, Wei LH. Effects of cupping on blood flow under skin of back in healthy human. World J Acupunct Moxibustion 2013;23(3):50-52.
- 10. Tagil SM, Celik HT, Ciftci S, Kazanci FH, Arslan M, Erdamar N, et al. Wet-cupping removes oxidants and decreases oxidative stress. Complement Ther Med 2014 Dec;22(6):1032-1036.
- 11. Bardaweel SK, Shehadeh M, Suaifan GA, Kilani MV. Complementary and alternative medicine utilization by a sample of infertile couples in Jordan for infertility treatment: clinics-based survey. BMC Complement Altern Med 2013 Feb;13(1):35-41.
- 12. Emerich M, Braeunig M, Clement HW, Lüdtke R, Huber R. Mode of action of cupping–local metabolism and pain thresholds in neck pain patients and healthy subjects. Complement Ther Med 2014 Feb;22(1):148-158.
- 13. Refaat B, El-Shemi AG, Ebid AA, Ashshi A, BaSalamah MA. Islamic wet cupping and risk factors of cardiovascular diseases: effects on blood pressure, metabolic profile and serum electrolytes in healthy young adult men. Altern Integr Med 2014;3(1):151.
- 14. Traditional and Complementary Medicine Division Ministry of Health Malaysia. [cited 2018 August 1]. Available from: http://tcm.moh.gov.my/en/.
- 15. Wan Nazaimoon WM, Md Isa SH, Wan Mohamad WB, Khir AS, Kamaruddin NA, Kamarul IM, et al. Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabet Med 2013 Jul;30(7):825-828.
- 16. Rampal S, Mahadeva S, Guallar E, Bulgiba A, Mohamed R, Rahmat R, et al. Ethnic differences in the prevalence of metabolic syndrome: results from a multi-ethnic population-based survey in Malaysia. PLoS One 2012;7(9):e46365.
- 17. Farahmand SK, Gang LZ, Saghebi SA, Mohammadi M, Mohammadi S, Mohammadi G, et al. The effects of wet cupping on coronary risk factors in patients with metabolic syndrome: a randomized controlled trial. Am J Chin Med 2012;40(2):269-277.
- 18. Ahmadi A, Schwebel DC, Rezaei M. The efficacy of wet-cupping in the treatment of tension and migraine headache. Am J Chin Med 2008;36(1):37-44.
- 19. Leaper D, Burman-Roy S, Palanca A, Cullen K, Worster D, Gautam-Aitken E, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BMJ 2008;337:a1924.
- 20. Al Showafi FK. Effect of blood cupping on some biochemical parameter. Med J Cairo Univ 2010;78(2):311-315.
- 21. Johnson RJ, Kang D-H, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003 Jun;41(6):1183-1190.
- 22. Kumaresan R, Giri P. A comparison of serum cystatin C and creatinine with glomerular filtration rate in Indian patients with chronic kidney disease. Oman Med J 2011 Nov;26(6):421-425.
- 23. Mula-Abed WA, Al Rasadi K, Al-Riyami D. Estimated glomerular filtration rate (eGFR): a serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med J 2012 Mar;27(2):108-113.
- 24. Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood 2015 Mar;125(13):2019-2028.
- 25. Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost 2006 Jun;4(6):1186-1193.
- 26. Mahdavi MRV, Ghazanfari T, Aghajani M, Danyali F, Naseri M. Evaluation of the effects of traditional cupping on the biochemical, hematological and immunological factors of human venous blood. A compendium of essays on alternative therapy Croatia: InTech 2012;67-88.