Polymyalgia rheumatica (PMR) is an inflammatory condition. Clinical features of PMR can present as complaints of rheumatic conditions like late-onset rheumatoid arthritis, giant cell arteritis, polymyositis, dermatomyositis, and other connective tissue diseases. Unlike connective tissue disease, lung involvement is a rare clinical feature in PMR. Clinically, the significance of dyspnea in those patients often attributed to generalized muscle weakness. The pulmonary function test in PMR is not fully studied.1 Similarly, conducting detailed radiographic investigation is not part of routine workup for patients presenting with features of PMR.
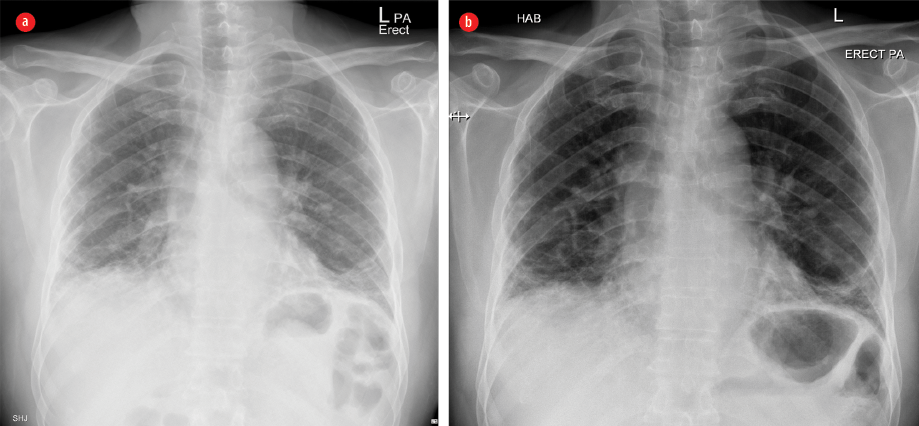
Figure 1: Plain X-ray frontal projection of the chest on admission (a) showing mildly diminished lung volume with interstitial opacities, mainly basal and subpleural in the form of reticular opacities with basal ground-glass veiling, patchy consolidation, and (b) three years after treatment showing relative regression of ground-glass veiling with persistence of the other features.
Case report
A 66-year-old, non-smoking male with a history of hypertension, diabetes mellitus, and ischemic heart disease presented to our hospital with a one-month history of exertional dyspnea, dry cough at rest, fatigue, muscular pain, and stiffness affecting the shoulder and hip girdles. His stiffness was worse in the morning, lasted for many hours, and rendered him unable to do activities of daily living. During this period, he had mood swings, which were noticed by his family members, and he lost around 5 kg of weight. There was no history of orthopnea or paroxysmal nocturnal dyspnea. There was no history of hemoptysis, wheeze, or other respiratory symptoms. Similarly, there was no history of visual disturbances or history suggestive of active arthritis.
Physical examination revealed a conscious, alert, oriented, and depressed elderly patient, who rated his pain as 5/10. His vital signs were normal, including a respiratory rate of 18 breaths per minute. Examination of the respiratory system revealed normal chest shape, normal thoracic spine, and no chest scar or deformity. Chest expansion was within the normal range, percussion note was resonant, and auscultation of the chest revealed symmetrical air entry of normal intensity, fine inspiratory crackles extending bilaterally over the infra-scapular region extending up to the interscapular region. The positive findings detected on the initial presentation were tenderness and decreased range of movements of the shoulder and hip girdles. Fundoscopy revealed chronic hypertensive changes and cotton wool spots. However, an examination of the other systems was normal, including the musculoskeletal system, as he did not have any features of active synovitis on clinical examination.
Table 1: Pulmonary function test (PFT) results at baseline and six months follow-up.
|
Vital capacity (VC), L |
1.98 |
2.99 |
|
Predicted VC, % |
75 |
75 |
|
FEV1/FVC ratio, % |
84 |
79 |
|
DLCO, % |
62 |
65 |
|
PH |
7.46 |
7.47 |
|
PO2, %mmHg |
72 |
82 |
|
PCO2, %mmHg |
35 |
32 |
FEV: forced expiratory volume; FVC: forced vital capacity; DLCO: diffusing capacity of lung for carbon monoxide.
His biochemical investigation results were as follows: hemoglobin 11.3 g/dL, white blood cell count 9.8 × 109/L, platelets 300 000 × 109/L, erythrocyte sedimentation rate (ESR) 65 mm/hour, creatinine kinase 32 U/L, and serum creatinine 0.9 mg/dL. Screening tests for autoimmune antibodies, including rheumatoid factor, citrulline antibody, anti-citrulline antibody, anti-nuclear antibody, and infection screen, including tuberculosis, were negative. An X-ray of his shoulders and hip joints were normal. Echocardiogram revealed concentric left ventricular hypertrophy, ejection fraction = 77%. The results of radiographic imaging can be seen in Figures 1 and 2, and the results of the pulmonary function test (PFT) are summarized in Table 1.
The patient was admitted with a working diagnosis of PMR associated with bronchiolitis obliterans organizing pneumonia (BOOP). We treated him symptomatically and kept him on prednisolone 40 mg daily for two weeks. His pain and stiffness diminished; he was able to do daily life activities, and his ESR dropped to 15 mm/hour. We maintained him on prednisolone 5 mg/day along with azathioprine 100 mg/day. His last visit to our clinic was in July 2017. In general, he felt well apart from mild dyspnea on exertion. Apart from fine end inspiratory crepitations which were heard over his chest, his physical examination was normal.
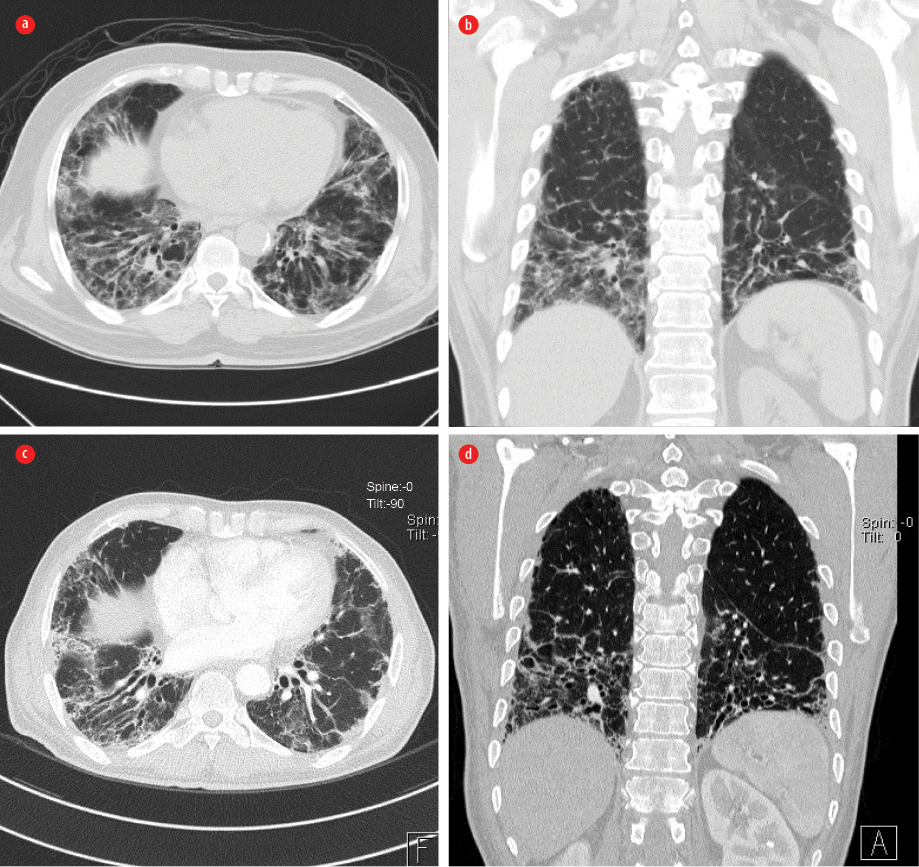
Figure 2: (a) Axial and (b) coronal computed tomography high resolution scans on admission showed mildly diminished lung volume with interstitial opacities, mainly in a basal and subpleural distribution, in the form of septal thickening, traction bronchiectasis, and small cystic changes matching with interstitial pulmonary fibrosis. Also noted was ground-glass veiling associated with patchy peripheral consolidation, which raises the suspicion of areas of bronchiolitis obliterans organizing pneumonia. (c) Axial and (d) coronal scans three years after treatment showed relative regression of both ground-glass appearance and the patchy consolidation.
Discussion
We have given an overview of the uncommon presentation of a case of PMR with lung involvement. PMR is an autoimmune disease characterized by stiffness of the neck, shoulder girdle, and pelvic girdle, whereas giant-cell arteritis (GCA) affects the cranial branches of arteries arising from the aortic arch. The two conditions are believed to be linked and may occur together.2 Approximately 50% of patients with GCA will have PMR, whereas 10% of PMR will have GCA.3,4 The diagnosis of PMR relies mainly on clinical features, and exclusion of inflammatory and non-inflammatory conditions, including GCA.5 The patient we are reporting had features in keeping with 2012 European League Against Rheumatism and the American College of Rheumatology (EULAR/ACR) provisional classification criteria for PMR.6 Our patient met the criteria for PMR classification as follows: age4 > 50 years (age 66 years), bilateral shoulder aching, and abnormal C-reactive protein and/or ESR. He also had all the clinical features supporting the classification of PMR, namely, morning stiffness duration of more than 45 minutes, hip pain or limited range of motion, absence of rheumatoid factor or anti-citrullinated protein antibodies, and absence of other joint pain. He had no visual disturbance nor temporal headache. Hence, there was no indication for temporal artery biopsy.
Lung involvement in PMR is usually uncommon. Few cohorts have described a spectrum of lung involvement in patients with PMR and GCA: pleural effusion, pulmonary artery vasculitis, pulmonary nodules, alveolitis, fibrosis, BOOP, malignancy, infection, and nodules. Indeed, those patients are at greater risk than their matched healthy controls for a chest infection and malignancy.7 The use of immunosuppressive therapy and the existence of co-morbidities further increases the risk of infection. Few cases in the literature have described the association of PMR and BOOP, similar to our case report.
Reports on the pattern of lung diseases detected on the PFT in patients with PMR are controversial. In a study that included 26 non-smoking patients with GCA and/or PMR and 28 sex- and age-matched non-smoker controls, 31% of those with PMR had normal PFT, and the rest had obstructive, restrictive and diffuse interstitial lung disease. In comparison, 50% of the control group had normal PFT.8 The study was not able to determine the clinical significance of PFT in patients with PMR. Our case showed a restrictive lung defect at presentation with mild improvement after six months, reflecting his clinical response.
In a retrospective study, two-thirds of patients with PMR had a significantly high frequency of computed tomography (CT) scan abnormalities compared to those with no PMR. These abnormalities included ground-glass opacity (56%), traction bronchiectasis (44%), architectural distortion (32%), centrilobular nodules (32%), and honeycombing (20%).9 In our patient, we saw features of interstitial pulmonary fibrosis and BOOP. A follow-up CT showed regression of BOOP features.
In general, PMR alone is a benign disease10 and usually has a very good prognosis if treated with steroids. The EULAR strongly advises against the use of steroids more than 30 mg.11 In this case, we tailored the therapy to 40 mg to target active PMR and BOOP. We introduced azathioprine as a steroid-sparing drug as the patients’ chest symptoms remained troublesome despite the improvement of his stiffness.
Conclusion
Lung involvement in PMR remains a clinical challenge in real-life as it determines the prognosis of the patient. Respiratory symptoms, including dyspnea and cough, can be easily overlooked and attributed to the patient’s immobility and generalized muscle weakness. Physicains should have a high index of suspicion when a patient with PMR presents with respiratory symptoms to rule out any lung involvement.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Sambataro G, Sambataro D, Pignataro F, Torrisi SE, Vancheri A, Pavone M, et al. Interstitial lung disease in patients with polymyalgia rheumatica: a case series. Respir Med Case Rep 2018 Dec;26:126-130.
- 2. Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002 Jul;347(4):261-271.
- 3. Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014 Jul;371(1):50-57.
- 4. Cantini F, Niccoli L, Storri L, Nannini C, Olivieri I, Padula A, et al. Are polymyalgia rheumatica and giant cell arteritis the same disease? Semin Arthritis Rheum 2004 Apr;33(5):294-301.
- 5. Al-Kaabi J, Ahmed S, Rizvi A, Burney I. Non-hodgkin lymphoma mimicking polymyalgia rheumatica in a young patient. Oman Med J 2008 Jul;23(3):189-191.
- 6. Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2012 Apr;71(4):484-492.
- 7. Ji J, Liu X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in patients hospitalized with polymyalgia rheumatica and giant cell arteritis: a follow-up study in Sweden. Rheumatology (Oxford) 2010 Jun;49(6):1158-1163.
- 8. Acritidis NC, Andonopoulos AP, Galanopoulou V, Drosos AA, Constantopoulos SH. Pulmonary function of nonsmoking patients with giant cell arteritis and/or polymyalgia rheumatica; a controlled study. Clin Rheumatol 1988 Jun;7(2):231-236.
- 9. Kinoshita S, Aoki T, Takahashi H, Oki H, Hayashida Y, Saito K, et al. Thin-section chest CT findings in polymyalgia rheumatica: a comparison between with and without rheumatoid arthritis. Clin Imaging 2016 May-Jun;40(3):382-385.
- 10. Ettlinger RE, Hunder GG, Ward LE. Polymyalgia rheumatica and giant cell arteritis. Annu Rev Med 1978;29(1):15-22.
- 11. Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, et al; European League Against Rheumatism; American College of Rheumatology. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015 Oct;74(10):1799-1807.