Thyroid cancer is the most common endocrine malignancy worldwide.1,2 it is ranked as the ninth most common cancer in both sexes and the fifth most common cancer affecting women.1 In 2018, the International Agency for Research on Cancer estimated that over half a million new cases of thyroid cancers (3.1% of all cancers) were diagnosed globally, causing more deaths than all other endocrine cancers combined (> 41 000 deaths, 0.4% of all cancer deaths).1 Data from as early as the 1940s suggested increasing temporal trends of thyroid cancer incidence in the US.3 Since, similar trends have been reported worldwide including the Americas, Asia, Europe, Oceania, and Nordic countries.4–7 More recent studies from Australia8 and the US9 confirmed increasing trends, and even one study described this increase as exponential.10
Thyroid cancer was the second most common cancer among women of the Arab Gulf Cooperation Council (GCC) states (Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, and UAE), with an average of 550 new cases (9.6% of total cancers in women) per year.11 In Oman, although incidence data on thyroid cancer are published annually by the national health authorities,12 its epidemiology and trends over the past two decades have not been studied closely. In this analysis, we present an outline of various patterns of thyroid cancer observed among Omani nationals from 1996 to 2015 and investigate the presence of trends, if any, during this period and projections of incidence rates over the next 20 years.
Methods
We extracted data of subjects diagnosed with thyroid cancer from the Oman National Cancer Registry (ONCR). Details of registry methods and data collection processes are published elsewhere.13 In brief, the registry collects its data by active (registry personnel visiting potential sources of case reporting and abstracting data on cancer registry forms) and passive data collection methods (case notification forms sent to the registry by the attending physician or medical record personnel). The variables collected include age, gender, date of diagnosis, morphology, topography, and tumor behavior with codes based on the third edition of the International Classification of Disease for Oncology (ICD-O3).14 Cases included in this analysis were subjects registered as having primary thyroid carcinomas (C73) from 1 January 1996 to 31 December 2015.
Our analysis was limited to Omani nationals in the ONCR database. Non-Omanis, mainly young male workers in Oman, represent a skewed population from a wide range of countries making the determination of a denominator for the calculation of incidence rates among them a complex exercise.
Frequency tables and crude incidence rates (CR) of thyroid cancer for five-year age groups by each calendar year and age-standardized incidence rates (ASR) were calculated using CanReg5 software.15 ASR was calculated using the World Standard Population, first proposed by Segi, and later modified by Doll and colleagues.16,17 The average CR and ASR over each of the three calendar periods (1996–2005, 2006–2015, and 1996–2015) were calculated using Microsoft Excel version 2013. The population denominators for the above three periods were obtained by averaging the population of the years 2000–2001, 2010–2011, and 2005–2006, respectively, to attain the mid-period population. Validity checks were performed for consistency between site/histology, gender/site, and age/site/histology combinations using inbuilt properties of CanReg5. Population data used for the calculations of CR by five-year age groups and gender were obtained from the Ministry of Health’s Annual Health Reports.18 Data were exported to Stata software (version 14.2, Stata Corporation, TX, USA) to obtain means, medians, and tumor morphology by gender. The p-values for the differences in ASR between males and females over the two periods (1996–2005 and 2006–2015) were generated using Poisson regression in Stata. To assess temporal trends in thyroid cancer by gender, we used the Joinpoint19 Regression Analysis program (with up to three joinpoints) to identify years (independent variable) at which statistically significant changes in ASR (dependent variable) occurred. Each joinpoint denotes a statistically significant change in trend.20 The size of this change is presented as an annual percentage change (APC) and the size of the overall change is presented as an average annual percentage change (AAPC) using the natural log-linear model, a Monte Carlo permutation test with Bonferroni adjustment for multiple comparisons and a p-value of < 0.050 as statistically significant.20 The heteroscedastic error option, during analysis, was set to select the standard error of the ASR as an estimate of the standard deviation. The calculated AAPC was applied to the average incidence rate over the study period to calculate projections for the subsequent 20 years, using the following formula:
Rate of subsequent year = (rate over study period) + (rate over study period × APC)
and the population attributable fraction (PAF) was obtained using the formula:
PAF = prevalance χ (relative risk-1)
(prevalence χ (relative risk-1))+1
to calculate the proportion of all thyroid cancers that can be attributed to a given exposure.
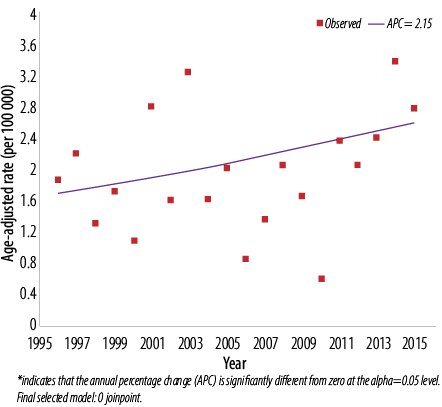
Figure 1: Trend of thyroid cancer in Omanis, 1996–2015. Males: 0 joinpoints.
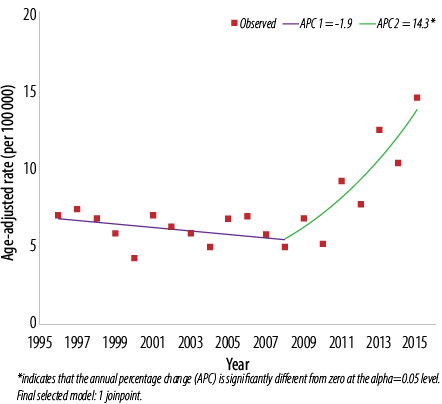
Figure 2: Trend of thyroid cancer in Omanis, 1996–2015. Females: 1 joinpoint.
Results
Thyroid cancer was the second most common cancer among Omani women and comprised 6.1% of all malignancies reported from 1996 to 2015. Out of the 1285 cases during this period, 80.9% (1040 cases) were among females, and 19.1% (245) were among males [Table 1]. Thyroid cancer did not reach the top 10 most frequent tumors affecting Omani men over the same period. Of the total, 62.0% of all cases in males and 67.0% of all cases in females were diagnosed in the second period (2006–2015), compared to 38.0% and 33.0% in the first period (1996–2005).
Table 1: Frequency, crude, and age-standardized rates of thyroid cancer in Oman by gender and time period, 1996–2015.
|
1996–2005 |
|
|
|
|
|
Male |
94 |
21.7 |
1.1 |
2.0 |
|
Female |
339 |
78.3 |
3.9 |
6.1 |
|
Total |
433 |
100 |
2.3 |
3.8 |
|
2006–2015 |
|
|
|
|
|
Male |
151 |
17.7 |
1.4 |
2.0 |
|
Female |
701 |
82.3 |
6.8 |
8.7 |
|
Total |
852 |
100 |
4.3 |
5.3 |
|
1996–2015 |
|
|
|
|
|
Male |
245 |
19.1 |
1.3 |
2.0 |
|
Female |
1040 |
80.9 |
5.5 |
7.6 |
|
Total |
1285 |
100 |
3.4 |
5.5 |
CR: crude incidence rates per 100 000 population; ASR: age-standardized incidence rates per 100 000 population.
The ASR from 1996 to 2015 ranged from 0.6 to 3.2 cases per 100 000 men [Figure 1] and 5.2 to 10.3 per 100 000 women [Figure 2]. When cases in the two calendar periods were compared, women tended to have between 3.0 to 4.5-time higher crude and ASR then men [Table 1]. There was no significant difference in the ASR of thyroid cancer in men between the first and second period (2.0 per 100 000 in both periods; p = 0.070), while in women, significantly higher ASR was observed in the second period compared to the first (8.7 per 100 000 vs. 6.1 per 100 000; p = 0.030). ASR was also significantly different between both genders over both periods (p < 0.010 for both periods).
Further assessment of temporal trends, using joinpoint regression analysis, showed no significant trend [Figure 1] in ASR in men (APC = 2.1%, 95% confidence interval (CI): -0.5% to 4.8%, p = 0.100) [Table 2]. In women, there was an initial decline in ASR from 1996–2008 followed by sharp and significant incline between 2008 and 2015 (APC = 14.3%, 95% CI: 8.0% to 20.9%, p < 0.010) [Figure 2]. In both genders, joinpoint analysis showed a significantly increasing trend with an overall AAPC of 3.7% (95% CI 0.2% to 7.4%; p < 0.010) over 20 years [Figure 3].
In both genders, 25.3% to 30.8% of thyroid cancer were diagnosed below the age of 30 years, and nearly 48.8% of women were diagnosed aged 30–49 years compared to 38.4% of men. Just 4.3% to 6.1% of men and women were diagnosed aged 75 years and above [Figure 4]. Women were younger at diagnosis, with the mean and median age of 38.5 and 36.0 years compared to 41.0 and 36.5 years in men, respectively. In both genders, ASR per 100 000 increased with increasing age and peaked in two age groups in women (45–50 years and 70–74 years) while in men, a single peak was observed (60–64 years) [Figure 5].
Papillary carcinoma was the dominant cancer type, and its proportion increased from 72.5% in the first period to 88.0% in the second period, while accounting for 83.0% of all tumors affecting thyroid over the 20 years. Follicular cancer formed 9.0% of all cases. Nearly all (99.0%) thyroid tumors were diagnosed through microscopic methods (histopathology) and 1.0% through all other methods (data not shown).
Table 2: Trends (joinpoint analysis) and average annual percentage change for thyroid cancer in Oman by gender, 1996–2015.
|
Males |
1996–2015 |
2.1 (-0.5 to 4.8;
p = 0.100) |
|
|
2.1 (-0.5 to 4.8;
p = 0100) |
|
Females |
1996–2008 |
-1.9 (-5.4 to 1.8;
p = 0.300) |
2008-2015 |
14.3* (8.0 to 20.9;
p < 0.010) |
3.8* (0.8 to 6.8;
p < 0.010) |
APC: annual percentage change; AAPC: average annual percentage change; CI: confidence interval.
*Indicated that the APC or AAPC are significantly different from zero at the alpha = 0.05 level.
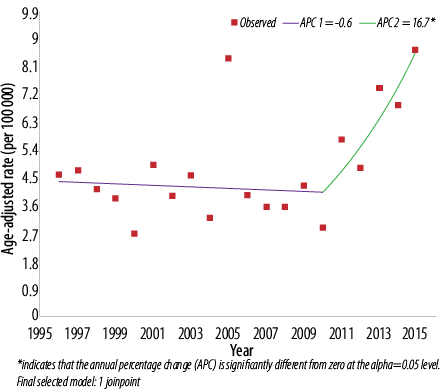
Figure 3: Trend of thyroid cancer in Omanis, 1996–2015. Both: 1 joinpoint.
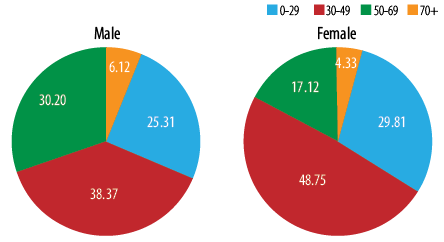
Figure 4: Proportion of thyroid tumors in Oman by gender and age group 1996–2015.
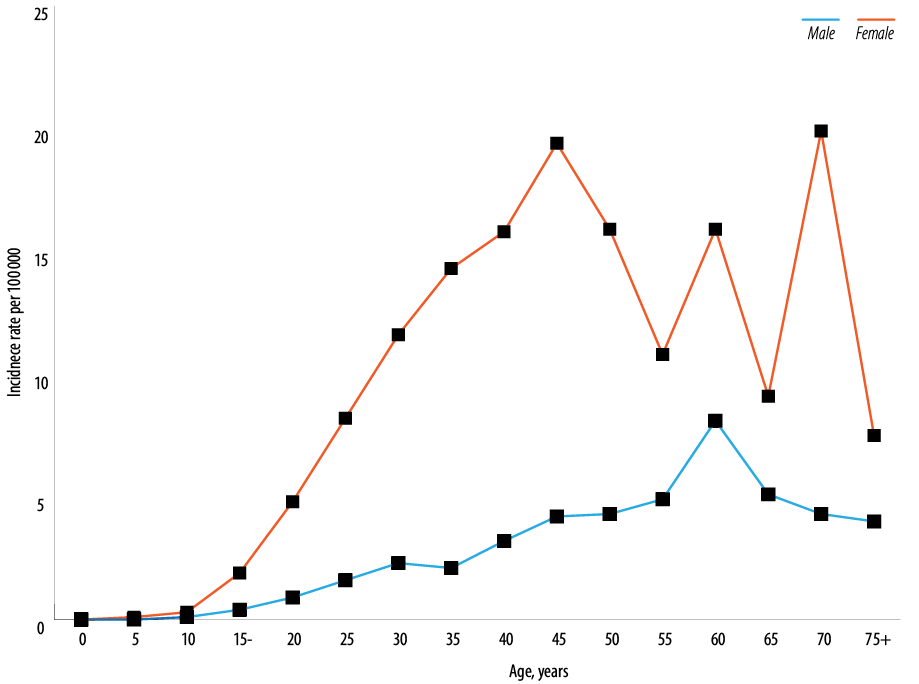
Figure 5: Age-specific incidence rates of thyroid cancer in Oman by gender, 1996–2015.
Discussion
Our study shows an increasing trend in the incidence of thyroid cancer in Oman between 1996 and 2015 in both women and men, although not statistically significant in men. The significant increase in the incidence in women and both genders combined was of similar magnitude, although the former was from 2008 to 2015, while the latter occurred from 2010 to 2015. This was consistent with findings from an earlier analysis of thyroid tumors in Oman between 1998 and 2012, reporting an increasing trend among women, albeit not statistically significant at the time.11 The difference could be due to the analysis technique used in this study (log-linear regression model) and the one used in the aforementioned study11 (linear regression model). On the other hand, this could be a true biological difference occurring over the two periods.
The highest age-adjusted incidence rates for thyroid cancer were reported from the Western Pacific region with South Korean men and women topping the world’s list (21.8 and 100.5, respectively), followed by women in Cyprus (33.4), Canada (30.1), Puerto Rico (29.6), French Polynesia (27.7), Costa Rica (23.6), Turkey (22.4), and the US (22.3), all per 100 000 population.21 On the other hand, the lowest ASR of thyroid cancer in men were reported from Tunisia, Libya, India, Kenya, and Togo (all < 1 per 100 000 population).21
Several studies worldwide reported increasing trends for thyroid cancer. A systematic review of 60 studies of trends in the incidence rates of thyroid cancer from Europe, North America, Asia, Oceania, and South America found 53 to have reported a significant increase in incidence (the highest was a 10-fold increase in South Korea), six reported stable rates, and one noted a decrease.22 A significantly increasing trend for thyroid cancer was also observed in the UAE and Saudi Arabia while increasing non-statistically significant trends were noted in Qatar and Kuwait, and a significantly decreasing trend in Bahrain.11
Although increasing trends for thyroid cancer have been detected worldwide,4,23 there is an ongoing controversy surrounding the likely reasons for this global increase. Some attribute this increase to “overdiagnosis” (that is, detection of small tumors, through extensive early detection/screening procedures, that would have never impaired health or required treatment).24 In 1999, the government and private health sector in South Korea encouraged the public to uptake early cancer detection, which was offered free of charge, or for a small fee of US$ 30–50 for people with above-average income.24 This led to widespread marketing of early detection programs with extensive use of ultrasonography, fine-needle aspiration biopsy, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) to screen for thyroid cancer.24 Two years later, the incidence rate of thyroid cancer in South Korea increased seven-fold compared to that observed in the base year of 1999.24 On the other hand, an analysis of trends for thyroid cancer in Australia estimated that no more than 50% of the increase in this malignancy could be attributed to increased use of ultrasonography, other imaging procedures, sensitive histopathologic techniques, and case ascertainment.25
Davies and Welch examined trends in thyroid cancer incidence and its size distribution and found that 49% of the increase was in tumors measuring ≤ 1 cm with 87% of tumors measuring ≤ 2 cm.26 They concluded that the increase in the incidence of thyroid cancer in the US was predominantly due to the increase in the detection of small papillary cancers, most likely caused by the widespread use of diagnostic procedures. More recent work, however, has shown that the increase in the incidence of thyroid cancer was not limited to small (≤ 1 cm) and localized tumors at the time of diagnosis, and a substantial increase was also observed for advanced stages as well as in larger (> 4 cm) size tumors.9,27 Thus, both studies rejected the notion that the surge in thyroid cancer incidence is merely due to ‘overdiagnosis’ and ‘surveillance bias’.9,27 Another large retrospective study reviewed 98 000 imaging scans of the head or neck, obtained by ultrasonography, CT, MRI, and PET scans and found only 0.4% of thyroid nodules were incidentally detected and ultimately reported, reflecting the minor contribution of such diagnostic procedures in the ‘overdiagnosis’ of thyroid cancer.28
Proponents of the ‘overdiagnosis’ concept also argued that the stability of thyroid cancer mortality that accompanied the increase in its incidence is clear evidence of the early detection-lead increase, rather than being a true biological phenomenon.24,26 However, two large scale analysis in the US recently showed that thyroid cancer mortality rates have significantly increased, in the first study by 0.8% and the second study by 1.1% per year overall and by 2.9% for those with advanced disease, and thus refuting the concept of ‘overdiagnosis’.9,29
In Oman, the increase in thyroid cancer occurred in the last eight years of the study period, with 99% of cases diagnosed through histopathological methods. The increase in papillary cancer was only 15.5% over the entire study period. There were also no early detection programs during the same period. Hence, we attribute the increase in trends of thyroid cancer in Oman to be a true biological increase, though some of this increase is likely to be due to the increased use of modern diagnostic procedures particularly ultrasonography which is nowadays widely available in all primary health care centers throughout Oman as well as the expansion of secondary and tertiary medical care institutions, not limited to the capital Muscat but throughout Oman from 1970 to current date. The contribution of ‘overdiagnosis’ to the increase in the incidence of thyroid cancer can only be determined with certainty by: a) assessing how each case came to medical attention, b) determining the proportion of tumors ≥ 4 cm diagnosed before and after the increase in incidence rates in 2008, and c) analysis of mortality records of thyroid cancer in Oman. All such variables were not available at the time of this study.
The magnitude of the increase in AAPC in thyroid cancer in our study was 2.1% in men, 3.8% in women, and 3.7% in both genders. Using these rates and assuming that trends will increase at a constant rate over the next 20 years, the ASR of thyroid cancer in men, women, and both genders are projected to reach 3.1, 16.6, and 11.8 per 100 000 by the year 2040. This will increase the burden on the current health system services which are likely to suffer due to a decline in government oil (9%) and other (15%) revenues30 financing many public service sectors including the national health service.
An increasing number of studies utilize joinpoint regression, a technique we used in this study to examine trends and quantify their magnitude. However, we could not find a comparable study from the Arab world, and the only study to use joinpoint regression analysis among Arabs was in Arab women living in Israel31 with an APC of 6.9%, lower than that observed in Oman. The reported APC in thyroid cancer in men and women for the US9was (3.1 and 3.7%), Australia8(5.5 and 6.1%), Sweden7 (0.8 and 7.6%) and Spain (5.4 and 4.7%), respectively.5 The APC among Jewish men and women in Israel were reported to be (4.0 and 4.2%), higher than the rates observed in Omani men (2.1%) but lower than in Omani women (14.2%), respectively.31 A study from China reported higher APC (20.0% in men and women) than in Oman.32
As modifiable risk factors for thyroid cancer are still ill-conceived, non-modifiable risk factors such as age, gender, ethnicity, and family history remain the strongest predictors of this disease.2 In our study, women had a nearly four-fold greater risk of thyroid cancer than men, highlighting gender as a strong risk predictor of this disease. Female preponderance is a worldwide phenomenon for thyroid cancer with 77–80% of cases occurring in women.1,2 A higher ASR of thyroid cancer was observed in women than men, respectively, from neighboring GCC countries11 like Bahrain (4.3 and 1.3), Qatar (6.3 and 1.3), Kuwait (12.9 and 1.8), Saudi Arabia (13.6 and 3.6), and the UAE (13.6 and 1.9), all per 100 000 population.21 Similar findings were also seen in populations from England,33 Scotland,6 Ireland,34 Spain,5 and elsewhere.21
The median age at diagnosis in Oman in women was 36.0 years compared to 36.5 years in men. In comparison, the median age at diagnosis for thyroid cancer in the US was 49 in women and 54 in men, and 45 for both ages in Ireland.2,35 It remains unclear as to why the risk in the Omani population starts almost a decade earlier than in other populations.
Among modifiable risk factors for thyroid cancer, obesity and not smoking tobacco were both reported to be positively associated with a higher risk of the disease.36 In Oman, the prevalence of obesity and non-smoking was estimated to be 20.5% and 92%, respectively.37,38 Assuming that the relative risks for thyroid cancer associated with obesity and non-current smoking2 are both 1.5 and that the observed associations with thyroid cancer risk are causal, then the proportion of all thyroid cancers that can be attributed to obesity and non-current smoking (PAF) would be approximately 9.3% and 31%, respectively, in Oman.
The strength of this study is that it is the first report to evaluate thyroid cancer epidemiology in Oman, its trends over 20 years using log-linear joinpoint regression models, incidence projections by gender for the next 20 years, and PAF for thyroid that is accounted by exposure to obesity and non-smoking status. Secondly, this study used good quality data generated by the national population-based registry covering the entire Omani population mostly through an active data collection system. However, our study also has few limitations. In addition to the wide range of data provided by the ONCR and analyzed therein, data on metastasis, regional distribution, modalities of treatment received by the patient, and mortality were not available from the database for comment. Thirdly, we did not have any data on the exposure of the Omani population to ionizing radiation, a crucial factor in the etiology of thyroid cancer.2
Conclusion
The incidence of thyroid cancer in Oman is of moderate rate compared to regional and international published figures, with evidence of an increasing trend in women but not in men. A significant amount of thyroid cancer can be prevented by controlling obesity, which requires the whole risk factor approach for control, not just for thyroid cancer, but also other chronic diseases that have obesity as an etiological factor.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018 Nov;68(6):394-424.
- 2. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016 Nov;12(11):646-653.
- 3. Devesa SS, Silverman DT, Young JL Jr, Pollack ES, Brown CC, Horm JW, et al. Cancer incidence and mortality trends among whites in the United States, 1947-84. J Natl Cancer Inst 1987 Oct;79(4):701-770.
- 4. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control 2009 Jul;20(5):525-531.
- 5. Salamanca-Fernández E, Rodriguez-Barranco M, Chang-Chan YL, Redondo-Sánchez D, Domínguez-López S, Bayo E, et al. Thyroid Cancer Epidemiology in South Spain: a population-based time trend study. Endocrine 2018 Nov;62(2):423-431.
- 6. Reynolds RM, Weir J, Stockton DL, Brewster DH, Sandeep TC, Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 2005 Feb;62(2):156-162.
- 7. Carlberg M, Hedendahl L, Ahonen M, Koppel T, Hardell L. Increasing incidence of thyroid cancer in the Nordic countries with main focus on Swedish data. BMC Cancer 2016 Jul;16:426.
- 8. Pandeya N, McLeod DS, Balasubramaniam K, Baade PD, Youl PH, Bain CJ, et al. Increasing thyroid cancer incidence in Queensland, Australia 1982-2008 - true increase or overdiagnosis? Clin Endocrinol (Oxf) 2016 Feb;84(2):257-264.
- 9. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017 Apr;317(13):1338-1348.
- 10. Hoang JK, Choudhury KR, Eastwood JD, Esclamado RM, Lyman GH, Shattuck TM, et al. An exponential growth in incidence of thyroid cancer: trends and impact of CT imaging. AJNR Am J Neuroradiol 2014 Apr;35(4):778-783.
- 11. Al-Madouj AN, Alothman SF, Eldali A, Al-Zahrani AS. 15-Year Cancer incidence among nationals of the GCC states 1998-2012. Riyadh: Gulf Center for Cancer Control and Prevention, King Faisal Specialist Hospital and Research Center, 2018.
- 12. Oman National Cancer Registry, General Directorate of Primary Health Care, Ministry of Health. Cancer Incidence in Oman [Cited 7 May 2019]. Available from: https://www.moh.gov.om/en/web/general-directorate-of-primary-health-care/resources
- 13. Al-Lawati JA, Al-Bahrani BJ, Al-Lawati NA, Al-Zakwani I. Epidemiology of Lung Cancer in Oman: 20-Year Trends and Tumor Characteristics. Oman Med J. 2019 Sep; 34(5): 397–403.
- 14. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al, eds. International Classification of Diseases for Oncology. Third Edition. Geneva: World Health Organization; 2000.
- 15. International Association of Cancer Registries, International Agency for Research on Cancer. CanReg5: Software for cancer registries [Cited 13 May 2019]. Available from: http://www.iacr.com.fr/index.php?option=com_content&view=category&layout=blog&id=68&Itemid=445.
- 16. Segi M. Cancer mortality for selected sites in 24 countries (1950-57). Sendai, Japan: Department of Public Health, Tohuku University of Medicine; 1960.
- 17. Doll R, Payne P, Waterhouse JA, eds. Cancer Incidence in Five Continents. Geneva: Union Internationale Contre le Cancer; 1966.
- 18. Ministry of Health. Oman. Annual Health Reports [cited 7 May 2019]. Available from: https://www.moh.gov.om/en/web/statistics/annual-reports.
- 19. National Cancer Institute. Statistical Research and Applications Branch, Joinpoint Program, Version 4.7.0.0. [computer program] 2019.
- 20. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000 Feb;19(3):335-351.
- 21. International Agency for Research on Cancer, The Union for International Cancer Control. Global Cancer Observatory [Cited 1 May 2019]. Available from: http://gco.iarc.fr/.
- 22. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid 2016 Nov;26(11):1541-1552.
- 23. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 2016 Aug;375(7):614-617.
- 24. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med 2014 Nov;371(19):1765-1767.
- 25. Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982-1997). Thyroid 2002 Feb;12(2):141-149.
- 26. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006 May;295(18):2164-2167.
- 27. Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009 Mar;18(3):784-791.
- 28. Uppal A, White MG, Nagar S, Aschebrook-Kilfoy B, Chang PJ, Angelos P, et al. Benign and Malignant Thyroid Incidentalomas Are Rare in Routine Clinical Practice: A Review of 97,908 Imaging Studies. Cancer Epidemiol Biomarkers Prev 2015 Sep;24(9):1327-1331.
- 29. Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2012 [Cited 26 May 2019]. Available from: https://seer.cancer.gov/archive/csr/1975_2012/.
- 30. KPMG. Oman budget 2019: KPMG Insights [Cited 25 May 2019]. Available from: https://assets.kpmg/content/dam/kpmg/ae/pdf/Oman-budget-2019.pdf.
- 31. Keinan-Boker L, Silverman BG. Trends of Thyroid Cancer in Israel: 1980-2012. Rambam Maimonides Med J 2016 Jan;7(1):7.
- 32. Du L, Li R, Ge M, Wang Y, Li H, Chen W, et al. Incidence and mortality of thyroid cancer in China, 2008-2012. Chin J Cancer Res 2019 Feb;31(1):144-151.
- 33. Finlayson A, Barnes I, Sayeed S, McIver B, Beral V, Ali R. Incidence of thyroid cancer in England by ethnic group, 2001-2007. Br J Cancer 2014 Mar;110(5):1322-1327.
- 34. National Cancer Registry. Cancer of the thyroid [Cited 23 May 2019]. Available from: https://www.ncri.ie/publications/cancer-trends-and-projections/cancer-trends-cancers-thyroid.
- 35. McVeigh TP, Mulligan RJ, McVeigh UM, Owens PW, Miller N, Bell M, et al. Investigating the association of rs2910164 with cancer predisposition in an Irish cohort. Endocr Connect 2017 Nov;6(8):614-624.
- 36. Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 2015 Dec;16(12):1042-1054.
- 37. Al-Lawati JA, Jousilahti PJ. Prevalence and 10-year secular trend of obesity in Oman. Saudi Med J 2004 Mar;25(3):346-351.
- 38. Ministry of Health. Oman. The Sultanate of Oman’s STEPS Survey 2017: Tobacco Fact Sheet. Preliminary results of the STEPS survey (unpublished data, available from the authors on request).