Ossification of the posterior longitudinal ligament (OPLL) is a hyperostotic condition characterized by hyperplasia of cartilage cells with eventual endochondral ossification, resulting in ectopic calcification of the posterior longitudinal ligament (PLL). The PLL is located within the vertebral canal and runs on the dorsal aspect of vertebral bodies and intervertebral discs of the vertebral column. It extends from the body of axis (C2) at the upper cervical spine down to the sacrum; above the level of the body of axis, it continues with the tectorial membrane. The ligament is broader at the cranial end than the caudal end and thicker in the thoracic region than the cervical and lumbar regions. It is wider at the level of intervertebral disc space and narrower at the level of vertebral bodies.1
OPLL was first recognized in the early 18th century. Initially, it was considered to occur only in Japan.2 Later, more OPLL related studies emanated from East Asia with comparatively fewer studies from other parts of the globe. OPLL is classified into four subtypes: continuous, segmental, localized, and mixed. In the continuous-type, ossification occurs over multiple segments continuously; in the segmental type, ossification occurs over the body of the vertebra; in the local type, ossification is observed only at the level of the intervertebral disc; and the mixed-type is a combination of the previous three types.3 OPLL appears in the form of a linear band of ossified tissue over the dorsal surface of the vertebral body and intervertebral discs.4 OPLL is commonly observed in the cervical spine, especially at the C2-C4 vertebral level.5 However, the occurrence of OPLL is also reported in the thoracic and lumbar regions.6 In clinical practice, computed tomography (CT) and magnetic resonance imaging (MRI) scans are frequently used to diagnose various spine-related conditions.7,8 Most recent studies have used CT or MRI for diagnosis.9–12 However, OPLL of cervical spine diagnosis using lateral X-rays can lead to underestimation of the condition, whereas MRI is prone to overestimation. This is because of the difficulty in distinguishing between hypertrophy and ossification. Therefore, CT has been demonstrated to be the best tool for diagnosis.13
OPLL pathogenesis and natural history are still unclear. Various factors (including genetic, environmental, lifestyle, and hormonal) are involved in the pathogenesis of OPLL.14–18 However, there is an ongoing debate on this issue. OPLL is usually associated with neurological defects and is also seen along with other diseases such as schizophrenia, ankylosing spondylitis, and diffuse idiopathic skeletal hyperostosis (DISH).19 In patients with schizophrenia, the incidence of OPLL is about 20%.20 Recently, an association between diabetes mellitus (DM) and OPLL has been demonstrated.21,22 An in vitro study has shown that hyperglycemia induces the synthesis of collagen from the cultured cells of cervical PLL through transforming growth factor-β1 and promotes hypertrophy of the ligament.23
The government of Oman provides free health services to all citizens. In Oman, as of now, there is no information on OPLL. In view of the various factors/conditions associated with OPLL, some of which are a burden in Oman, our study was undertaken to help generate baseline data on the proportion of OPLL of the cervical spine among patients referred for CT scan in a tertiary care referral hospital in Oman, and to study its association with age, gender, and DM.
Methods
Our study included both admitted patients and out-patients aged ≥ 20 years who had visited the Sultan Qaboos University Hospital (SQUH) and were referred to the Radiology Department for CT scans of the cervical region between May 2011 and August 2017. All patients with a history of spinal fracture, previous spinal surgery, and non-Omani nationals were excluded from the study. The patients’ data were collected from the database of the Radiology Information System and Hospital Information System of SQUH, which was the sole source of the study. Patient demographics such as age, gender, nationality, and diabetic status were documented. The diagnosis of OPLL was made based on the findings of CT scans, covering the region from the clivus to vertebra prominence (C7) using PACS® software (version 4.4.516.21, Philips, Intellispace, USA). After diagnosis, patterns and the vertebral levels of ossification were noted manually from each scanned image [Figure 1].3 Reviews of all CT images were done by the same radiologist. The study was reviewed and approved by the Medical Research Ethics Committee, College of Medicine, SQUH (Ref. no. SQU-EC/154/17).
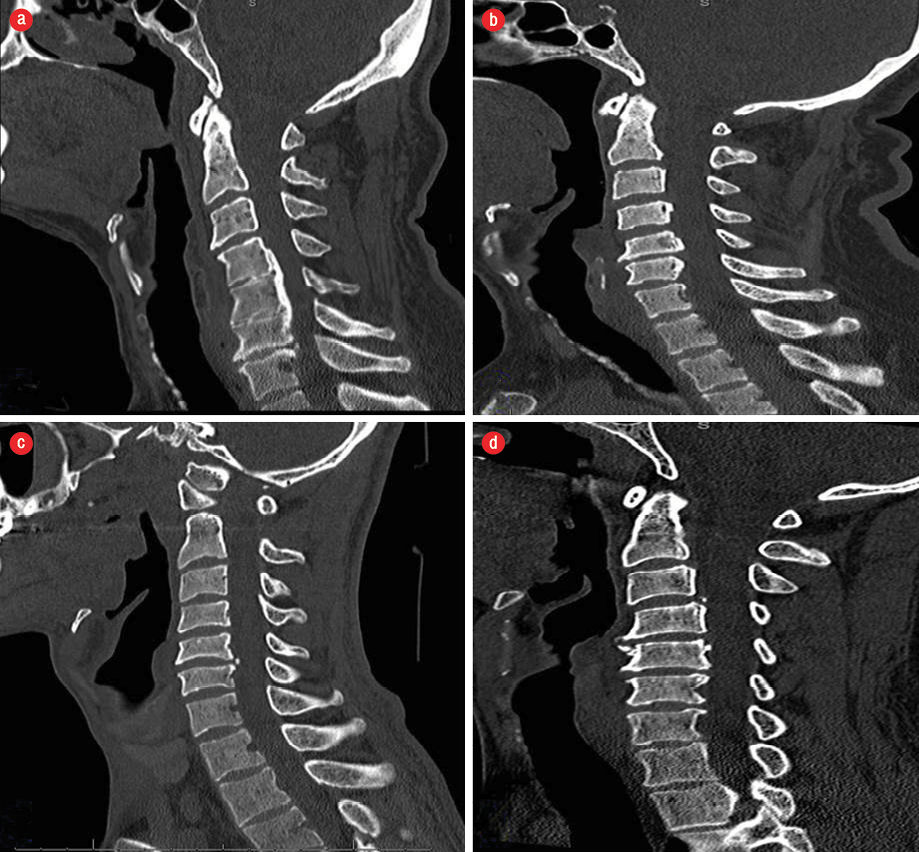
Figure 1: CT scans of different ossification of the posterior longitudinal ligament types: (a) continuous,
(b) segmental, (c) localized, and (d) mixed.
SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IB) was used to analyze the data. Descriptive statistics were used to present the data. The association of OPLL with age, gender, and the presence of DM was determined using the chi-square test. A p-value of less than 0.050 was considered to be statistically significant.
Results
A total of 843 patients were included in the study. Among these patients, 533 were men and 310 were women. The proportion of OPLL was 2.7% (n = 23) and was more frequent in men (3.2%, n = 17) than women (1.9%, n = 6). The male to female ratio of OPLL was 1.7:1.0. Among the different types of OPLL, the segmental and the localized were the most commonly observed types (10 each). The continuous and the mixed types were observed only in two and one cases, respectively. The most frequent type of OPLL was localized among men (n = 8) and segmental among women (n = 4) [Table 1 and Figure 1]. The continuous and mixed types were not observed in women. OPLL occurrence was significantly more in non-diabetics (5.9%) than in patients with DM (1.2%, p < 0.001). No significant association was found between OPLL with gender (p = 0.281) and age (p = 0.878) [Table 2]. The majority of localized type cases were noted at the C3/C4 (n = 4) and C5/C6 (n = 5) vertebral levels [Table 3]. The segmental type of OPLL cases was observed at the level of all the cervical vertebrae except the first cervical vertebra.
Table 1: Proportion of patients with ossification of the posterior longitudinal ligament (OPLL) by gender and age.
|
Males |
21–30 |
148 |
0 |
2 |
1 |
0 |
151 |
|
31–40 |
128 |
1 |
0 |
4 |
0 |
133 |
|
41–50 |
79 |
0 |
1 |
1 |
0 |
81 |
|
51–60 |
121 |
1 |
3 |
1 |
0 |
126 |
|
61–70 |
38 |
0 |
0 |
1 |
1 |
40 |
|
> 70 |
2 |
0 |
0 |
0 |
0 |
2 |
|
Total |
516 |
2 |
6 |
8 |
1 |
533 |
|
21–30 |
62 |
0 |
1 |
0 |
0 |
63 |
|
31–40 |
84 |
0 |
1 |
0 |
0 |
85 |
|
41–50 |
64 |
0 |
2 |
1 |
0 |
67 |
|
51–60 |
61 |
0 |
0 |
0 |
0 |
61 |
|
61–70 |
27 |
0 |
0 |
1 |
0 |
28 |
|
> 70 |
6 |
0 |
0 |
0 |
0 |
6 |
Discussion
Our study determined the proportion of cases of OPLL among Omani patients as 2.7%. These findings are marginally higher than the findings from other Asian ethnic groups noted in studies from Hong Kong (0.8%),24 Singapore (0.8%),25 the Philippines (1.5%),26 and Korea (0.6–1.9%).27,28 However, our findings are similar to studies from Japan (1.9–4.3%)26 and Taiwan (2.6–7.7%).26,29
Table 2: Association between ossification of the posterior longitudinal ligament (OPLL) with gender, age, and diabetes status.
|
Gender |
|
|
|
|
Males |
516 |
17 |
NS |
|
Females |
304 |
6 |
|
|
Age, years |
|
|
|
|
21–30 |
210 |
4 |
|
|
31–40 |
212 |
6 |
|
|
41–50 |
143 |
5 |
|
|
51–60 |
182 |
5 |
NS |
|
61–70 |
65 |
3 |
|
|
> 70 |
8 |
0 |
|
|
DM |
|
|
|
|
No |
254 |
16 |
< 0.001 |
NS: not significant; DM: diabetes mellitus.
Table 3: Vertebral levels of ossification of the posterior longitudinal ligament (OPLL).
|
Continuous |
C4-C6 |
1 |
|
C5-C6 |
1 |
|
Segmental |
C5 |
1 |
|
C3,C4 |
1 |
|
C5,C6 |
3 |
|
C2,C3,C4 |
1 |
|
C3,C4,C5 |
1 |
|
C4,C5,C6 |
1 |
|
C4,C5,C6,C7 |
1 |
|
C2,C3,C4,C5,C6 |
1 |
|
Localized |
C3/C4 |
4 |
|
C5/C6 |
5 |
|
C6/C7 |
1 |
Initially, OPLL was thought to be unique to the Asian population, and due to this there was little research in the US and Europe.30 Later, OPLL research gained momentum in western populations when studies revealed the incidence of OPLL in Caucasians having DISH.31 The results of this research emphasized that OPLL is a DISH subtype. In West Germany and the US, the observed prevalence of cervical spine OPLL ranged from 0.01–1.7%.26 In a study conducted among different ethnic groups, the prevalence of cervical OPLL has been reported as 1.3% in Caucasian Americans and 4.8% in Asian Americans.32 These results further confirm the previously proposed hypothesis that Asians are more likely to be affected by ectopic OPLL compared to non-Asians.
Different risk factors, such as DM, age, genetic factors, and environmental factors, are associated with OPLL.14–18 DM was found to be an independent risk factor for the onset of OPLL.21,22 A high prevalence of DM was noted in patients with cervical myeloradiculopathy due to OPLL.33 Further, a positive correlation between ossified spinal ligament and glycation end products was observed.34 Our findings from this limited study are similar to other studies,35,36 but are in contrast to the established positive association of OPLL and DM.21,22,33,34
Age is an independent risk factor for the onset of OPLL.22,37 In our sample, OPLL was more prevalent in the 31–40 year age group, although this was not statistically significant. Our findings are in line with the majority of studies which report male predominance in OPLL with the male/female ratio varying between 1.1 and 3.0.29,37 However, one study noted opposite findings of 1:3 ratio, and this discrepancy could be due to sampling bias and the method of screening.38 In Japanese39 and Koreans,27 the most frequent location of OPLL was the vertebral levels of C4, C5, C6 and C3, C4, C5, respectively. In our study, OPLL was more frequently observed at the vertebral levels of C3, C4, C5, and C6.
We could not investigate the genetic relationship with OPLL even though the prevalence of genetic diseases in Oman is high.40 There is a possibility of underestimation of the prevalence due to selection bias as we included only those subjects who underwent radiological investigation at this center.
Conclusion
The observed proportion of OPLL in this single-center study is relatively low, but the finding is important as the condition can eventually lead to debilitating neurologic outcomes affecting the quality of life of the patient. OPLL occurrence was significantly more in non-diabetic patients, which indicates the need for further research on OPLL in a larger sample across Oman.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
We thank Dr. M. Inguva, an English language expert from Language Center, Sultan Qaboos University, Oman for her help in language editing of our manuscript.
references
- 1. Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976) 2012 Mar;37(5):E309-E314.
- 2. Miyazaki K, Kirita Y. Extensive simultaneous multisegment laminectomy for myelopathy due to the ossification of the posterior longitudinal ligament in the cervical region. Spine (Phila Pa 1976) 1986 Jul-Aug;11(6):531-542.
- 3. Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Global Spine J 2016 Mar;6(2):195-204.
- 4. Choi BW, Song KJ, Chang H. Ossification of the posterior longitudinal ligament: a review of literature. Asian Spine J 2011 Dec;5(4):267-276.
- 5. Nagata K, Sato K. Diagnostic imaging of cervical ossification of the posterior longitudinal ligament. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL. 2nd ed. Tokyo: Springer; 2006. p. 127-143.
- 6. Ohtsuka K, Terayama K, Yanagihara M, Wada K, Kasuga K, Machida T, et al. A radiological population study on the ossification of the posterior longitudinal ligament in the spine. Arch Orthop Trauma Surg 1987;106(2):89-93.
- 7. Islam SM, Abru AF, Al Obaidani S, Shabibi SA, Al Farsi S. Trends in CT request and related outcomes in a pediatric emergency department. Oman Med J 2016 Sep;31(5):365-369.
- 8. Sirasanagandla SR, Al Dhuhli H, Al Abri A, Salmi A, Jayapal SK, Sara C, et al. Prevalence of diffuse idiopathic skeletal hyperostosis among elderly subjects referred for radiological investigation in tertiary hospital at Oman. Anat Cell Biol 2018 Sep;51(3):174-179.
- 9. Kim KW, Oh YM, Eun JP. Increased prevalence of ossification of posterior longitudinal ligament and increased bone mineral density in patients with ossification of nuchal ligament. Korean J Spine 2016 Sep;13(3):139-143.
- 10. Izumi T, Hirano T, Watanabe K, Sano A, Ito T, Endo N. Three-dimensional evaluation of volume change in ossification of the posterior longitudinal ligament of the cervical spine using computed tomography. Eur Spine J 2013 Nov;22(11):2569-2574.
- 11. Wang MY, Thambuswamy M. Ossification of the posterior longitudinal ligament in non-Asians: demographic, clinical, and radiographic findings in 43 patients. Neurosurg Focus 2011 Mar;30(3):E4.
- 12. Lin D, Ding Z, Lian K, Hong J, Zhai W. Cervical ossification of the posterior longitudinal ligament: Anterior versus posterior approach. Indian J Orthop 2012 Jan;46(1):92-98.
- 13. Kobashi Y, Munetomo Y, Baba A, Yamazoe S, Mogami T. Evaluation of the ossification of the cervical posterior longitudinal ligament utilizing X-Ray, CT and MR imaging. Orthop Res Traumatol Open J 2017;2(1):35-39.
- 14. Koga H, Sakou T, Taketomi E, Hayashi K, Numasawa T, Harata S, et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet 1998 Jun;62(6):1460-1467.
- 15. Maeda S, Koga H, Matsunaga S, Numasawa T, Ikari K, Furushima K, et al. Gender-specific haplotype association of collagen alpha2 (XI) gene in ossification of the posterior longitudinal ligament of the spine. J Hum Genet 2001;46(1):1-4.
- 16. Kon T, Yamazaki M, Tagawa M, Goto S, Terakado A, Moriya H, et al. Bone morphogenetic protein-2 stimulates differentiation of cultured spinal ligament cells from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 1997 Mar;60(3):291-296.
- 17. Kawaguchi Y, Furushima K, Sugimori K, Inoue I, Kimura T. Association between polymorphism of the transforming growth factor-beta1 gene with the radiologic characteristic and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2003 Jul;28(13):1424-1426.
- 18. Song J, Mizuno J, Hashizume Y, Nakagawa H. Immunohistochemistry of symptomatic hypertrophy of the posterior longitudinal ligament with special reference to ligamentous ossification. Spinal Cord 2006 Sep;44(9):576-581.
- 19. Sakai K, Okawa A, Takahashi M, Arai Y, Kawabata S, Enomoto M, et al. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: a prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine (Phila Pa 1976) 2012 Mar;37(5):367-376.
- 20. Matsunaga S, Koga H, Kawabata N, Kawamura I, Otusji M, Imakiire T, et al. Ossification of the posterior longitudinal ligament in dizygotic twins with schizophrenia: a case report. Mod Rheumatol 2008;18(3):277-280.
- 21. Moon BJ, Choi SK, Shin DA, Yi S, Kim KN, Yoon DH, et al. prevalence, incidence, comorbidity, and mortality rates of ossification of posterior longitudinal ligament in the cervical spine: a nested case-control cohort study. World Neurosurg 2018 Sep;117:e323-e328.
- 22. Kobashi G, Washio M, Okamoto K, Sasaki S, Yokoyama T, Miyake Y, et al; Japan Collaborative Epidemiological Study Group for Evaluation of Ossification of the Posterior Longitudinal Ligament of the Spine Risk. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine (Phila Pa 1976) 2004 May;29(9):1006-1010.
- 23. Li H, Liu D, Zhao CQ, Jiang LS, Dai LY. High glucose promotes collagen synthesis by cultured cells from rat cervical posterior longitudinal ligament via transforming growth factor-β1. Eur Spine J 2008 Jun;17(6):873-881.
- 24. Kurokawa T. Prevalence of ossification of the posterior longitudinal ligament of the cervical spine in Taiwan, Hong Kong, and Singapore. Investigation Committee 1977 report on the ossification of the spinal ligaments of the Japanese Ministry of Public Health and Welfare; 1978. p. 8-9.
- 25. Lee T, Chacha PB, Khoo J, Khoo J. Ossification of posterior longitudinal ligament of the cervical spine in non-Japanese Asians. Surg Neurol 1991 Jan;35(1):40-44.
- 26. Kim TJ, Bae KW, Uhm WS, Kim TH, Joo KB, Jun JB. Prevalence of ossification of the posterior longitudinal ligament of the cervical spine. Joint Bone Spine 2008 Jul;75(4):471-474.
- 27. Shin J, Kim YW, Lee SG, Park EC, Yoon SY. Cohort study of cervical ossification of posterior longitudinal ligament in a Korean populations: Demographics of prevalence, surgical treatment, and disability. Clin Neurol Neurosurg 2018 Mar;166:4-9.
- 28. Matsunaga S, Sakou T. OPLL: disease entity, incidence, literature search, and prognosis. Tokyo: Springer; 2006. p. 11-17.
- 29. Wu JC, Liu L, Chen YC, Huang WC, Chen TJ, Cheng H. Ossification of the posterior longitudinal ligament in the cervical spine: an 11-year comprehensive national epidemiology study. Neurosurg Focus 2011 Mar;30(3):E5.
- 30. Matsunaga S, Sakou T. Epidemiology of ossification of the posterior longitudinal ligament. In: Yonenobu K, Sakou T, Ono K, editors. OPLL. Tokyo: Springer; 1997. p. 11-17.
- 31. McAfee PC, Regan JJ, Bohlman HH. Cervical cord compression from ossification of the posterior longitudinal ligament in non-orientals. J Bone Joint Surg Br 1987 Aug;69(4):569-575.
- 32. Fujimori T, Le H, Hu SS, Chin C, Pekmezci M, Schairer W, et al. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine (Phila Pa 1976) 2015 Apr;40(7):E394-E403.
- 33. Tauchi R, Lee S-H, Peters C, Imagama S, Ishiguro N, Riew KD. Cervical myeloradiculopathy due to ossification of the posterior longitudinal Ligament with versus without diffuse idiopathic spinal hyperostosis. Global Spine J 2016 Jun;6(4):350-356.
- 34. Yokosuka K, Park JS, Jimbo K, Yoshida T, Yamada K, Sato K, et al. Immunohistochemical demonstration of advanced glycation end products and the effects of advanced glycation end products in ossified ligament tissues in vitro. Spine (Phila Pa 1976) 2007 May;32(11):E337-E339.
- 35. Chin DK, Han IB, Ropper AE, Jeon YJ, Kim DH, Kim YS, et al. Association of VKORC1-1639G>A polymorphism with susceptibility to ossification of the posterior longitudinal ligament of the spine: a Korean study. Acta Neurochir (Wien) 2013 Oct;155(10):1937-1942.
- 36. Wang PN1, Chen SS, Liu HC, Fuh JL, Kuo BI, Wang SJ. Ossification of the posterior longitudinal ligament of the spine. A case-control risk factor study. Spine (Phila Pa 1976). 1999 Jan 15;24(2):142-4; discussion 145.
- 37. Ikegawa S. Genomic study of ossification of the posterior longitudinal ligament of the spine. Proc Jpn Acad Ser B Phys Biol Sci 2014;90(10):405-412.
- 38. Hiramatsu Y, Nobechi T. Calcification of the posterior longitudinal ligament of the spine among Japanese. Radiology 1971 Aug;100(2):307-312.
- 39. Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res 1984 Apr;(184):71-84.
- 40. Rajab A, Al Salmi Q, Jaffer J, Mohammed AJ, Patton MA. Congenital and genetic disorders in the Sultanate of Oman. First attempt to assess healthcare needs. J Community Genet 2014 Jul;5(3):283-289.