Non-Hodgkin’s lymphomas (NHLs) are known to affect lymphoid tissues in the Waldeyer’s ring. Typical histology of nasopharyngeal lymphoma is diffuse large B-cell lymphoma. Mantle cell lymphoma (MCL) is an aggressive subtype of NHL, and only constitutes 3–4% of all Waldeyer’s ring lymphomas1 and 5% of all NHLs.2 The median age of the diagnosis is 68 years old, and MCL has a male-to-female predilection ratio of 2.5:1.2
To our knowledge, no more than five cases of nasopharyngeal MCL have been reported in the English literature, and none of the cases reported relapse to involve the nasopharynx.1–5
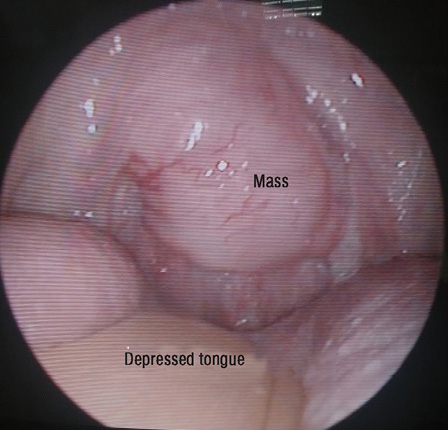
Figure 1: Tongue depressed with wooden spatula revealing soft tissue mass extending from the nasopharynx and involving uvula.
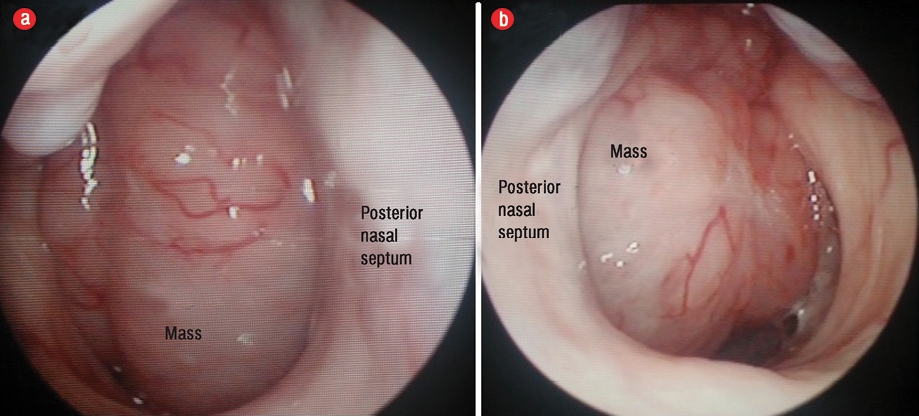
Figure 2: Nasopharyngeal mass completely obstructing the (a) right posterior choana and (b) left posterior choana.
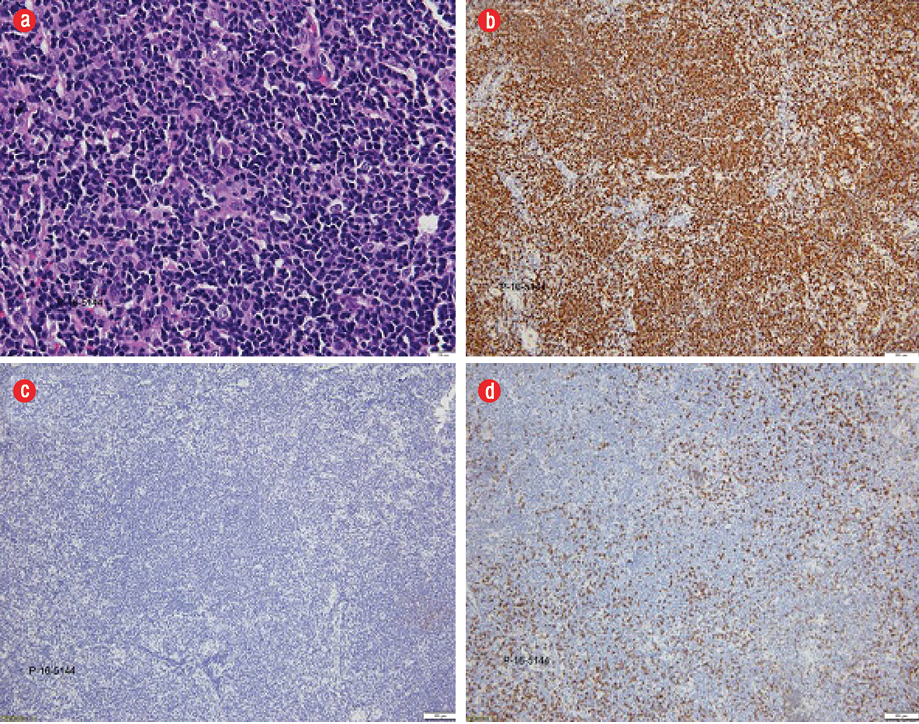
Figure 3: (a) Hematoxylin and eosin staining revealed atypical lymphoid cells of medium size with a round to oval shape with vesicular nuclei and irregular nuclear membrane, magnification = 400 ×. (b) The atypical lymphoid cells were positive for CD20 (brown) immunohistochemical stain, magnification = 40 ×. A similar positivity pattern was seen for CD79a, CD5, and cyclin D1 (images not shown). (c) The atypical lymphocytes were negative for CD3 immunohistochemical stain, magnification = 40 ×. A similar negativity pattern for CD10 and CD23 was seen (images not shown). (d) Ki-67 staining (brown) revealed a proliferative index of 20–30%, magnification = 40 ×.
Case Report
A 66-year-old man with diabetes and hypertension presented with a seven-month history of loose stool, rectal bleeding, abdominal pain, and fever lasting two days. He was diagnosed with a grade one hemorrhoid. Colonoscopy revealed a polypoidal mass obstructing the whole bowel lumen with features of intussusception 120 cm from the anal verge. The patient subsequently underwent exploratory laparotomy and extended right hemicolectomy with primary anastomosis. Intraoperative findings revealed colon intussusception at hepatic flexure. Histopathology examination revealed abdominal MCL. Staging computed tomography scan established a stage three disease based on Ann Arbor classification without nasopharyngeal involvement. Positron emission tomography scan was not done as it was not available in the region. Bone marrow aspiration was normal. He received two cycles of RCEOP (rituximab, cyclophosphamide, etoposide, vincristine, and prednisolone) and one cycle of RDHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin, and prednisolone) chemotherapy before it was truncated due to recurrent diabetic foot ulcer and acute coronary syndrome.
During a routine follow-up session at the hematology clinic two months later, a mass was seen at the posterior oropharyngeal wall. There were no abdominal masses or complaints. He was referred to an otolaryngologist for further assessment.
He did not experience any nasal blockage, epistaxis, neck swelling, ear symptoms, or swallowing difficulties. Oral examination revealed a single soft mass extending from nasopharynx involving the uvula at the soft palate [Figure 1]. His tonsils were not enlarged. Nasoendoscopy revealed a smooth-surfaced, highly vascular mass occupying the whole nasopharynx, completely obstructing right posterior nasal choana [Figure 2a], and 90% of the left posterior nasal choana [Figure 2b]. Biopsy of the mass revealed lymphoid tissue completely effaced by medium-sized lymphoid cells and irregular nuclear membrane. Those lymphoma cells expressed diffuse positive immunohistochemical stain for CD20, CD79a, CD5, and cyclin D1, but were negative for CD10, CD23, BCL6, and MUM1. Proliferative index (Ki-67) was about 20–30% without overt blastic change seen [Figure 3]. He was diagnosed with MCL relapse at the nasopharynx. Creatine kinase, lactate dehydrogenase, and other blood parameters were normal.
Unfortunately, the patient is currently under palliative care as his comorbidities rendered therapeutic measures unsuitable. He was seen six months after initial detection of the nasopharyngeal mass. The mass remained the same size.
Discussion
MCL is one of the less common B-cell subtypes of NHL and develop from non-germinal center B-cells within the mantle zone of the lymph nodes and lymphoid tissues.1 The underlying cytogenetics is due to translocation t(11;14)(q13;q21), which eventually results in overexpression of cyclin D1, a cell cycle protein not usually expressed in lymphoid cells; therefore, disrupting the central cell cycle pathway balance resulting in MCL.1
The disease has an aggressive clinical course, but rare indolent cases have been reported.3,6 Patients usually present at later stages of the disease with extensive lymph nodes, liver, spleen, and bone marrow involvement.5 Like NHL, staging of this illness is done using the Ann Arbor system. Approximately 5% to 15% of patients will present with limited stage I and II disease.6 Frequent relapse after completing chemotherapy has been reported.1–3,5 Due to the mentioned reasons, MCL has a poor prognosis.
Several cases of gastrointestinal tract extranodal MCL have been reported, presenting with multiple polyps (lymphomatous polyposis) causing single or multi-level intussusceptions like in our patient.1,4,6 Some of those cases had regional recurrences after surgery and chemotherapy, but to the best of our knowledge, this is the first case with relapse involving the nasopharynx.
Circulating mantle cells can be seen in the peripheral blood film, but blood investigation in our patient was unremarkable.6
Overall survival of MCL with conventional chemotherapy at 10 years was 8% at an American cancer center.6 Intensive chemotherapy which consists of intensive rituximab together with fractionated hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) regimen alternating every 21 days with rituximab plus high-dose methotrexate and cytarabine for three or four cycles of both regimens revealed 82% overall survival at three years.6
A combination of chemotherapy with optional rituximab followed by autologous stem cell transplantation showed positive results (83% overall survival at five years).6 Radiation therapy may benefit limited stage I and II disease as MCL is radiosensitive (70% overall survival at five years).6
Conclusion
Nasopharyngeal MCL is very rare and thus a standard treatment regime does not exist for this entity. New and improved treatment regimens for MCL are being formulated. Early diagnosis and commencement of treatment are crucial factors pertaining to disease aggressiveness and improving survival rates.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Tan LH. Lymphomas involving Waldeyer’s ring: placement, paradigms, peculiarities, pitfalls, patterns and postulates. Ann Acad Med Singapore 2004 Jul;33(4)(Suppl):15-26.
- 2. Zhou Y, Wang H, Fang W, Romaguer JE, Zhang Y, Delasalle KB, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer 2008 Aug;113(4):791-798.
- 3. Koletsa T, Markou K, Ouzounidou S, Tsiompanou F, Karkavelas G, Kostopoulos I. In situ mantle cell lymphoma in the nasopharynx. Head Neck 2013 Nov;35(11):E333-E337.
- 4. Lin MT, Kuo YH, Chuah SK, Chiu YC, Huang CC, Tai WC, et al. Mantle cell lymphoma with diffuse gastrointestinal tract involvement: A case report. J Intern Med Taiwan 2009;20:555-560.
- 5. Kang JH, Park YD, Lee CH, Cho KS. Primary mantle cell lymphoma of the nasopharynx: a rare clinical entity. Braz J Otorhinolaryngol 2015 Jul-Aug;81(4):447-450.
- 6. Straus DJ. How I treat mantle cell lymphoma. J Oncol Pract 2007 Sep;3(5):281-282.