Leukocytoclastic vasculitis may be idiopathic or associated with drugs, infections, malignancies, and connective tissue disorders. Infections linked to causing small vessel vasculitis include streptococcal and staphylococcal infections, tuberculosis, leprosy, hepatitis B, hepatitis C, HIV, and infective endocarditis.
We report the case of a 30-year-old man with visceral leishmaniasis (kala-azar) and leukocytoclastic vasculitis. The patient improved symptomatically following treatment with amphotericin B. There are rare case reports of visceral leishmaniasis occurring in patients diagnosed vasculitis. Cutaneous vasculitis per se has not been reported with visceral leishmaniasis, especially at disease presentation and thus, could be a possible red herring in timely diagnosis and treatment.
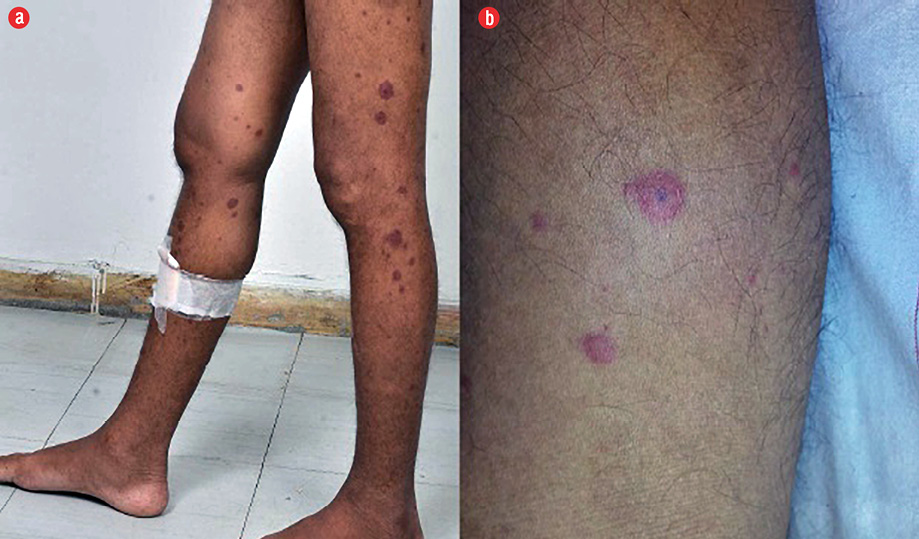
Figure 1: (a) Clinical photograph of the lower limbs showing numerous erythematous palpable purpura predominantly spread over the extensor surface. (b) Magnified view of the cutaneous lesion showing raised erythematous purpura with a dark necrotic center.
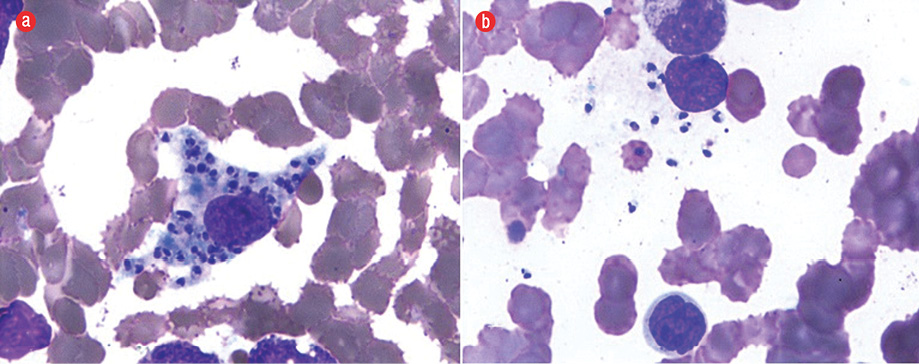
Figure 2: Presence of (a) intracellular and (b) extracellular Leishman-Donovan bodies. Giemsa staining, magnification = 1000 ×.
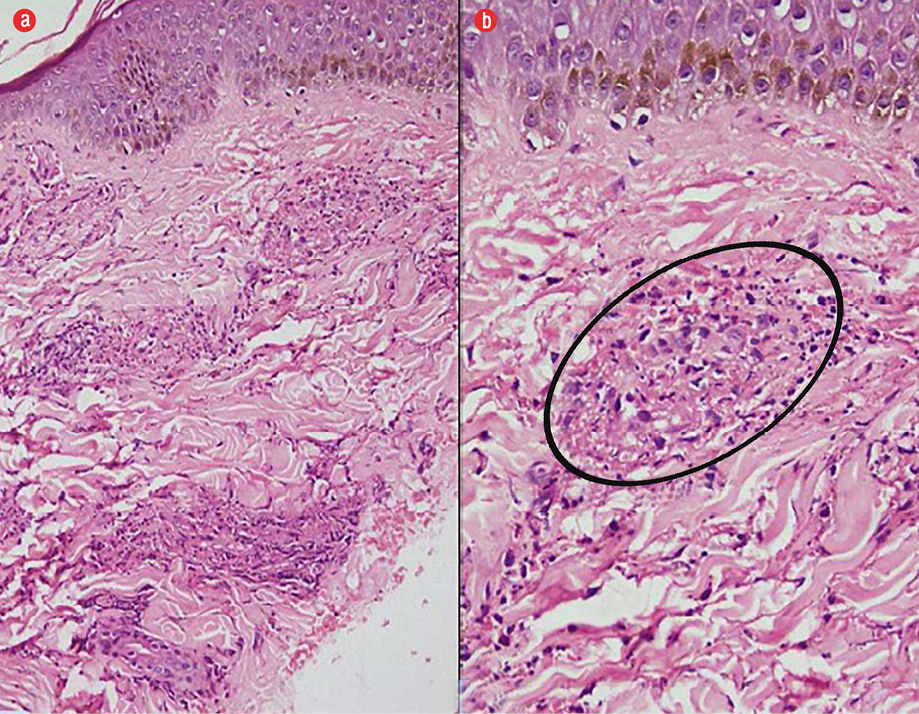
Figure 3: (a) Hematoxylin and eosin staining of skin biopsy samples revealing leukocytoclastic vasculitis with extensive fibrinoid necrosis within dermal capillaries, magnification = 200 ×. (b) Fibrinoid necrosis destroying the vessel wall with extravasation of red blood cells and nuclear debris in the adjacent areas, magnification = 400 ×.
Case Report
A 30-year-old man presented with pain in his lower limbs and non-itchy, erythematous rash over both lower limbs. He had low-grade intermittent fever of three-months duration. On examination, the patient was pale and had palpable purpura over both thighs and buttocks [Figure 1]. He was malnourished (body mass index = 18.4 kg/m2), and abdominal examination revealed palpable hepatomegaly with massive splenomegaly.
A complete blood count suggested pancytopenia (hemoglobin = 6.8 g/dL, total leukocyte count = 2800/mm3, and thrombocyte count = 45 000/μL). Polyclonal hypergammaglobulinemia (4.9 g/dL) was noted with normal liver and renal function tests. Contrast-enhanced computed tomography of the chest and abdomen confirmed hepatosplenomegaly and was otherwise unremarkable.
We considered hematological malignancy or a connective tissue disorder with vasculitis. Other infectious etiologies entertained were HIV, scrub typhus rickettsia, and visceral leishmaniasis (given the patient lived in an endemic area). Anti-nuclear antibodies, complement levels (C3 = 94 mg/dL and C4 = 20 mg/dL), and rheumatoid factor were negative. Urine examination was normal and serum cryoglobulins were not raised. Viral markers (HIV, hepatitis B surface antigen, and anti-hepatitis C) were negative. Serology for rickettsia and scrub typhus were also negative. Peripheral blood antibodies towards rK39 antigen were positive, and bone marrow examination confirmed visceral leishmaniasis with the presence of typical Leishman-Donovan bodies (amastigote form) within the bone marrow histiocytes [Figure 2]. Skin biopsy of the rashes revealed fibrinoid necrosis of the vessel wall along with extravasation of red blood cells and nuclear debris in the adjoining areas consistent with a diagnosis of leukocytoclastic vasculitis [Figure 3].
Conventional amphotericin B (0.7 mg/kg/day) was given for 14 days with close monitoring of electrolytes, renal function, and urine output along with adequate nutritional support. During treatment, the patient’s fever resolved, his blood counts improved, and we noted complete resolution of skin rash.
Discussion
Visceral leishmaniasis is a tropical systemic infection caused by protozoa of the genus Leishmania (Leishmania donovani in Asia and Africa, Leishmania infantum in the Mediterranean, and Leishmania chagasi in South America). The sand fly (Phlebotomus) is the vector for transmission.
The occurrence of the disease in any region with an altitude greater than 2000 feet above sea level is rare. But in a recent study from a tertiary center in Himachal Pradesh, India, 18 patients with kala-azar were admitted over a five-year period, of which eight patients hailed from the Kinnaur district (7612 to 22 362 feet),1 confirming the endemicity of the area.
Clinical manifestations are due to the damage to the organs of the mononuclear phagocytic cell system, mainly in the spleen and may vary from nonfatal cutaneous lesions to lethal visceral involvement. However, the diagnosis is established by demonstrating the parasite in tissue or by serology. Splenic aspirates are useful for parasite demonstration,2 but the lack of expertise and the presence of thrombocytopenia in most patients makes bone marrow examination the preferred modality. Amastigotes within the bone marrow smear are diagnostic with 100% specificity but have a sensitivity of only 60–85%.2 Sensitivity and specificity of antibodies to recombinant antigen rK39 are 91.9% and 92.4%, respectively.3
Liposomal amphotericin B is the only drug approved (by the US Food and Drug Administration) for the treatment of visceral leishmaniasis. The conventional preparation amphotericin B deoxycholate is the preferred drug in resource limited establishments, while pentavalent antimonials are the first choice outside Asia.
Small vessel vasculitis of the skin is mediated by immune complex deposition in the affected vessels. Circulating antigens due to drugs, infections, connective tissue disorders, or neoplasm bind to the antibodies and complexes are deposited in the vessels of the superficial dermis. Among the infectious causes of cutaneous small vessel vasculitis, beta-hemolytic streptococcus and hepatitis C are commonly implicated. HIV and bacterial endocarditis are also known to cause leukocytoclastic vasculitis. Tuberculosis (pulmonary and extrapulmonary) has been linked to leukocytoclastic vasculitis.4 Visceral leishmaniasis in canines has been associated with systemic vasculitis,5 and cryoglobulinemic vasculitis has been reported in humans with visceral leishmaniasis.6 However, the association of visceral leishmaniasis with secondary infection-related cutaneous small vessel vasculitis has not been described. Patients may also develop vasculitic lesions as a result of drugs (especially antibiotics) given to treat infections. In such situations, determining the cause of vasculitis may be difficult.
Palpable purpura is a characteristic of vasculitic process involving small vessels. Index case had biopsy-proven vasculitic rash limited to the involvement of cutaneous system.
Cutaneous vasculitis secondary to drugs or infection usually resolves with drug cessation or treatment of the underlying infection. Dapsone and corticosteroids are recommended for patients with refractory lesions or serious systemic manifestations.7,8
Temporal relation with the diagnosis of kala-azar and complete resolution with amphotericin B treatment suggests a secondary involvement due to infection. Extensive workup of the index case ruled out systemic vasculitis and majority of the other mentioned infectious causes of leukocytoclastic vasculitis. Prompt resolution with chemotherapy makes a coincidental idiopathic cutaneous vasculitis unlikely. Since visceral leishmaniasis is not a common cause of cutaneous vasculitis, the treating physician might miss the diagnosis of this treatable condition in favor of other etiologies.
Conclusion
Leukocytoclastic small vessel dermal vasculitis may be idiopathic or secondary to drugs, infections, malignancies and connective tissue disorders. Infections causing secondary small vessel cutaneous vasculitis are streptococcus, staphylococcus, tuberculosis, leprosy, hepatitis B, hepatitis C, HIV, and infective endocarditis. Visceral leishmaniasis maybe a potential cause of infection-associated cutaneous vasculitis. The presence of vasculitic skin lesions should not deter the clinician from suspecting leishmaniasis, a fully treatable infectious cause, especially in patients from endemic regions.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Raina S, Mahesh DM, Kaul R, Satindera KS, Gupta D, Sharma A, et al. A new focus of visceral leishmaniasis in the Himalayas, India. J Vector Borne Dis 2009 Dec;46(4):303-306.
- 2. Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 2002 Sep;9(5):951-958.
- 3. Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database Syst Rev 2014 Jun;(6):CD009135.
- 4. Kim HM, Park YB, Maeng HY, Lee SK. Cutaneous leukocytoclastic vasculitis with cervical tuberculous lymphadenitis: a case report and literature review. Rheumatol Int 2006 Oct;26(12):1154-1157.
- 5. Pumarola M, Brevik L, Badiola J, Vargas A, Domingo M, Ferrer L. Canine leishmaniasis associated with systemic vasculitis in two dogs. J Comp Pathol 1991 Oct;105(3):279-286.
- 6. Casato M, de Rosa FG, Pucillo LP, Ilardi I, di Vico B, Zorzin LR, et al. Mixed cryoglobulinemia secondary to visceral Leishmaniasis. Arthritis Rheum 1999 Sep;42(9):2007-2011.
- 7. Fredenberg MF, Malkinson FD. Sulfone therapy in the treatment of leukocytoclastic vasculitis. Report of three cases. J Am Acad Dermatol 1987 Apr;16(4):772-778.
- 8. Micheletti RG, Werth VP. Small vessel vasculitis of the skin. Rheum Dis Clin North Am 2015;41(1):21-32, vii.