In in vitro fertilization (IVF) cycles, it is important to have appropriate controlled ovarian stimulation (COS) to compensate for the shortcomings in embryo culture and transfer cycles and increase the rate of implantation, pregnancy, and live births.1 Patients who respond poorly to the traditional COS protocols are considered poor responders whose prevalence is between 5.6% and 35.1% in different series and based on the various available criteria.2,3 Poor response to COS is associated with an increased number of cycles required to retrieve appropriate embryos, decreased number of retrieved oocytes, reduced available embryos for transfer, and decreased conception and live birth rates.4–6 Several protocols and methods have been introduced for treatment of poor responders with different results,7–12 but there is not enough evidence to support any intervention or protocol for treatment of these patients.
Age, antral follicle count (AFC), and anti-Müllerian hormone (AMH) levels are among the most important predictors of poor response to COS.13 In other words, decreased ovarian reserve is the main pathophysiologic mechanism of poor response to COS. Additionally, variations in gonadotropin receptor regulations and presentations are associated with suboptimal response.14 Although several mechanisms have been described for variations in response to COS, the exact pathophysiology is yet to be identified. The variations in response to COS could be due to the fact that there are many follicles in each ovary in different developmental stages with various follicle-stimulating hormone (FSH) receptors. Recruitment of these follicles results in different responses to COS because of various FSH receptors.13 The other explanation is that during the late menstrual cycle, FSH concentration increases dramatically to preserve the antral follicles, while luteinizing hormone (LH) decreases.15 Those follicles that are more sensitive to the lower concentrations of FSH (due to their genetic factors), develop during the late luteal phase, and thus lead to different follicular size during the early days of subsequent menstrual cycle resulting in asynchronous response to COS.16
All protocols used for poor responders are focused on minimizing the early follicular growth in the luteal phase and normalizing hormonal variations in the follicular phase to obtain an optimal follicular response to COS. This is the rationale of using oral contraceptives or gonadotropin-releasing hormone (GnRH) antagonists at the end of the luteal phase to suppress FSH and prevent premature follicular development. A novel protocol entitled “delayed start” was described for improving the follicular response to COS.8 In this protocol, delaying the start of COS with GnRH antagonists for seven days after the estrogen rise resulted in suppression of FSH in the early follicular phase and thus resulted in synchronous follicular development.8 However, this theory was tested in a cohort study and further evidence is required to confirm the results. We performed this randomized clinical trial to determine the effects of the delayed start protocol with GnRH antagonist in poor responders undergoing IVF.
Methods
This randomized, double-blinded clinical trial was conducted during a 15-month period from April 2014 to July 2015 in the IVF clinic of the Mother and Child Hospital, a tertiary health care center affiliated with Shiraz University of Medical Sciences, Iran. The study protocol was approved by the institutional review board and medical ethics committee of Shiraz University of Medical Sciences. All patients provided their informed written consent before inclusion in the study. The study protocol was registered with the Iranian Registry for Clinical Trials (IRCT2015040821657N2; www.irct.ir). We included infertile poor responder women referred to our center during the study period. All included patients suffered from primary infertility with no previous live births. Poor response to COS was defined according to the Bologna poor responder criteria17 in which at least two of the following three features must be present: (i) advanced maternal age or any other risk factor for poor ovarian response, (ii) a previous poor ovarian response, and (iii) an abnormal ovarian reserve test. Those with severe male factor infertility, uterine myoma, uterine anomaly, and hydrosalpinx were excluded from the study.
All recruited patients were randomly assigned to two study groups using a computerized random digit generator using the patient admission number, which were provided consecutively. Those assigned to the study group underwent IVF cycles using the delayed start GnRH antagonist protocol and those in the control group underwent the standard COS protocol. Delayed start protocol was defined as administration of cetrorelix acetate (Cetrotide®, Injection, Powder, 250 µg, Serpero pharmaceutical, Italy) as a GnRH antagonist at a dose of 250 µg per night on cycle day two to seven, and those in the control group underwent standard COS by GnRH antagonist protocol with estradiol priming. All patients and those who administered the intervention were blinded to the study groups. Those measuring the outcomes were also blinded to the groups. Only statisticians were aware of the study groups.
All patients received estrogen priming using estrogen pills (Premarin, 0.625 mg Tablets, Ferrer Inc., Madrid, Spain) from day 21 of the cycle and continued to menstruation. The absence of ovarian cyst and dominant follicle > 10 mm was confirmed by performing ultrasonography on day two of the cycle and after completion of the GnRH antagonist protocol. In the control group, COS was started by gonadotropins on day two of the menstrual cycle. In the study group (delayed start protocol), COS was performed seven days after GnRH antagonist pretreatment with administration of 250 µg Cetrotide per night on cycle day two to seven. In control group, COS was induced by administration of 300 IU FSH (Gonadal F; Merck Inc., Germany) and 150 IU human menopausal gonadotrophin (hMG; Menopur; 75 IU Ampules, Ferring Inc., Germany). They also received Cetrotide with the same dosage from day nine of the cycle. In those with lead follicle measuring ≥ 12 mm, we added Cetrotide to prevent the premature LH surge. Ovarian maturation was induced by injection of 10 000 IU of human chorionic gonadotropin (hCG; Ferring Inc., Germany) after detecting antral follicle > 18 mm in diameter. Oocyte pick-up was performed in those that had at least two follicles measuring > 18 mm or three follicles measuring > 13 mm. The cycle was canceled in those who did not meet the criteria, and they underwent intrauterine sperm injection. Oocyte retrieval was performed 36 hours after injection of hCG under the guide of ultrasonography through the vagina under general anesthesia. Then intracytoplasmic sperm injection (ICSI) was performed using fresh, ejaculated sperm and mature, metaphase II (MII) stage oocytes. ICSI was performed in all cycles to decrease the failure rate. The embryos were transferred into the uterine cavity after fertilization on day two or three based on the number of embryos.
The primary endpoint of the study was considered to be the number of patients who underwent oocyte pick-up (defined as those with at least two follicles > 18 mm and three follicles > 13 mm). The secondary outcome measures included the rates of implantation, pregnancy, the numbers of total oocytes, retrieved mature oocytes, dominant follicles (> 13 mm) on the day of hCG trigger, retrieved oocytes, transferred embryos, the oocyte maturity rate (number of MII follicles/total number of oocytes), oocyte yield (total number of oocytes retrieved/AFC), mature oocyte yield (number of mature oocytes retrieved/AFC), number of days needed for ovarian stimulation, and serum levels of LH and FSH. We also recorded the number of patients who qualified for oocyte retrieval.
To have 80% power to detect a 5% difference between the two study groups regarding the primary endpoint, by considering the rate of pregnancy rate in previous studies,8 a total of 19 patients was required in each study group. To compensate for non-evaluable patients, we included 21 patients in each group. All statistical analyses were performed using SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Data are presented as mean±standard deviation (SD) and proportions as appropriate. Parametric variables with normal distribution were compared using the independent t-test between the two study groups while proportions were compared using the chi-square test. Paired t-test was used to compare the data within groups. Parametric variables without a normal distribution were compared using the Kruskal-Wallis test. We also ran a multivariate logistic regression model to control for confounding factors. A two-sided p-value of < 0.050 was considered statistically significant.
Results
We screened 51 individuals for eligibility of whom 42 were randomized into two study groups (each containing 21 patients). All patients in the traditional COS protocol finished the study and none were lost to follow-up. However, two patients in delayed start protocol discontinued the intervention. Thus the total number of patients included in the final analysis was 40 (19 in delayed start protocol and 21 in the traditional COS protocol) [Figure 1]. The baseline characteristics of the patients were comparable between the two study groups [Table 1].
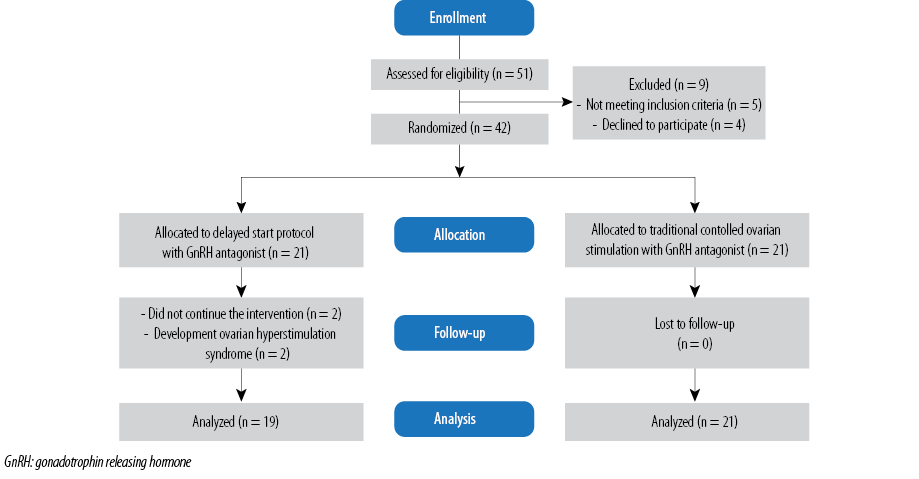
Figure 1: CONSORT flow diagram of the study.
Table 1: Baseline characteristics of 40 infertile patients undergoing controlled ovarian stimulation using the delayed start and traditional protocols.
|
Age, years |
37.8 ± 4.0 |
36.6 ± 5.2 |
0.436 |
|
Body mass index, kg/m2 |
27.8 ± 3.7 |
27.1 ± 2.6 |
0.227 |
|
Infertility duration, months |
46.8 ± 18.1 |
47.2 ± 21.9 |
0.198 |
|
Previous IVF cycle, n (%) |
1 (5.3) |
0 (0.0) |
0.475 |
Data given as mean±standard deviation unless otherwise indicated.
AFC: antral follicle count; IVF: in vitro fertilization.
Table 2: The outcome of in vitro fertilization cycles in 40 patients with infertility undergoing controlled ovarian stimulation using the delayed start and traditional protocols.
|
Ovarian stimulation duration, days |
10.8 ± 2.3 |
11.9 ± 1.8 |
0.543 |
|
Endometrial thickness, mm |
11.3 ± 2.5 |
10.6 ± 3.4 |
0.078 |
|
FSH, mIU/mL |
8.6 ± 2.8 |
9.3 ± 3.5 |
0.680 |
|
AMH, ng/mL |
0.9 ± 0.7 |
1.03 ± 0.8 |
0.463 |
|
Follicle > 13 mm |
4.5 ± 2.7 |
2.9 ± 2.5 |
0.057 |
|
Total unit of FSH |
5214.3 ± 1631.2 |
5863.1 ± 1527.9 |
0.068 |
|
Total oocyte retrieved |
3.0 ± 1.2 |
2.4 ± 1.9 |
0.564 |
|
MII oocyte retrieved |
2.5 ± 1.2 |
1.7 ± 2.4 |
0.366 |
|
MII/total oocytes ratio |
0.8 ± 0.3 |
0.7 ± 0.2 |
0.569 |
|
Oocytes/AFC ratio |
0.4 ± 0.4 |
0.3 ± 0.4 |
0.472 |
|
MII oocytes/AFC ratio |
0.4 ± 0.4 |
0.2 ± 0.3 |
0.238 |
|
Number of embryos |
1.8 ± 2.0 |
2.2 ± 4.7 |
0.709 |
|
Embryos transferred |
1.3 ± 1.3 |
0.6 ± 1.1 |
0.060 |
|
Number of patients undergoing oocyte pick-up |
15 (78.9) |
13 (61.9) |
0.311 |
|
Implantation rate, n (%) |
3 (15.8) |
2 (9.5) |
0.407 |
Data given as mean±standard deviation unless otherwise indicated.
FSH: follicle-stimulating hormone; AMH: anti-Mullerian hormone; AFC: antral follicle count; MII: metaphase II.
There was no significant difference between the two study groups regarding the duration of COS (p = 0.543), endometrial thickness (p = 0.078), serum levels of FSH (p = 0.680), and AMH (p = 0.463) [Table 2]. The number of follicles measuring > 13 mm in diameter was slightly higher in the delayed start protocol group compared to the traditional protocol (p = 0.057). The number of retrieved oocytes (p = 0.564), mature oocytes (p = 0.366), embryos (p = 0.709), and transferred embryos (p = 0.060) were comparable between the two study groups. With regard to the final outcome measures, the number of patients undergoing oocyte pick-up (p = 0.311), implantation rate (p = 0.407), and the pregnancy rate (p = 0.596) were also comparable between the two groups. We ran a multivariate logistic regression model to control for confounding factors such as age, body mass index (BMI), and infertility duration; none of the primary and secondary outcome measures were significantly different between the two groups.
Discussion
We investigated the effects of the delayed start protocol on the outcome of poor responders undergoing IVF through a randomized clinical trial. We found that COS using a delayed start protocol was not associated with alleviated IVF outcomes when compared to the traditional protocol. These findings are contrary to those previously reported, which showed the delayed start protocol was associated with improved ovarian response in poor responders.8 The authors of the study reported that synchronizing follicle development (without impairing oocyte developmental competence) is the main mechanism of this novel protocol.8 However, their study was a retrospective cohort with a limited number of patients. They also included only those patients who failed in their estrogen priming antagonist protocol, which resulted in selection bias. In addition, patients included in the study had one previous estrogen priming failed cycle.8 The initial poor response in the conventional antagonist cycle could be idiosyncratic and resulted in improved outcomes in the delayed start protocol. However, in this randomized clinical trial, we demonstrated that delayed start protocol was not associated with improved cycle outcome in poor responders when compared to traditional protocols.
The main pathophysiology of poor response to COS is decreased ovarian reserve determined by decreased AFC and increased AMH, which is associated with infertility and limited response to standard COS protocols.18 The quality of the oocytes and produced embryos is also diminished in poor responders which lead to aggravated outcomes in these groups.19 The standard protocols of COS using GnRH antagonists are effective in those patients not classified as poor responders.20–23 The management of poor responders is focused on increasing the number of oocytes and embryos in order to increase the implantation and birth rate.24 The economic burden and length of COS are significantly higher in poor responders and the outcome worse.25 In addition, several strategies have been introduced and applied for poor responders to alleviate the IVF or ICSI cycles with controversial results.5,7,8,11,18,26–28 The hypothesis of delayed start protocol is that estrogen pretreatment in the preceding luteal phase and an immediate and short pituitary shutdown with GnRH antagonist in the early follicular phase increases the rate of oocyte maturation by providing more time for FSH receptors to develop, and thus provide better response to the pituitary hormones resulting in better development of oocytes.
A previous study showed that this protocol improves the outcome both theoretically and functionally.8 But, in a randomized clinical trial, we observed no difference between the standard protocols and the delayed start protocol regarding the fertilization indices.
Suppression of FSH in the early follicular phase lead to improved maturation and development of follicles.29 In GnRH antagonist protocols, a higher concentration of gonadotropins is available before the start of COS.30 This results in higher serum levels of FSH before starting COS which in turn causes maturation and growth of the leading follicles before adding the exogenous FSH. Early gonadotropin suppression during the subsequent follicular phase could be easily achieved by several options including estrogen, GnRH antagonists, or oral contraceptives.31,32 Rapid suppression of FSH in early follicular phase using GnRH antagonists may also contribute to improved maturation and growth of the lead follicle.33
We note some limitations to our study. First, we included a limited number of poor responders. However, the power of the study was 80%, and both study groups included the minimal required number of participants (n = 19). Our results warrant further multicenter randomized clinical trials including larger numbers of patients. Second, we did not measure the serum estradiol level. This could help us to determine the response to COS in poor responders undergoing the delayed start or traditional protocol and their association with fertility outcome of the cycles. To the best of our knowledge, this is the only randomized clinical trial addressing the issue, and further studies are required.
Conclusion
The delayed start protocol was not associated with better conception results or cycle outcome in poor responders with primary infertility undergoing IVF cycles. Further multicenter clinical trials with larger study populations are required to shed light on the issue.
Disclosure
The authors declared no conflicts of interest. This study was funded by a grant from vice chancellor of research of Shiraz University of Medical Sciences (grant number 95-207).
Acknowledgements
We would like to thank all the patients and their families who participated in this study. We would also thank all the personnel of the infertility clinics of Mother and Child Hospital of Shiraz. We acknowledge the editorial assistant of Diba Negar Research Institute for improving the style and English of the manuscript.
references
- 1. Alper MM, Fauser BC. Ovarian stimulation protocols for IVF: is more better than less? Reprod Biomed Online 2017 Apr;34(4):345-353.
- 2. Biljan MM, Buckett WM, Dean N, Phillips SJ, Tan SL. The outcome of IVF-embryo transfer treatment in patients who develop three follicles or less. Hum Reprod 2000 Oct;15(10):2140-2144.
- 3. Hendriks DJ, te Velde ER, Looman CW, Bancsi LF, Broekmans FJ. Expected poor ovarian response in predicting cumulative pregnancy rates: a powerful tool. Reprod Biomed Online 2008 Nov;17(5):727-736.
- 4. Ulug U, Ben-Shlomo I, Turan E, Erden HF, Akman MA, Bahceci M. Conception rates following assisted reproduction in poor responder patients: a retrospective study in 300 consecutive cycles. Reprod Biomed Online 2003 Jun;6(4):439-443.
- 5. Ahmad G, Lewis-Jones DI, Gazvani R. Prolonged ovarian hyperstimulation for poor responders in in-vitro fertilization treatment cycles. Int J Gynaecol Obstet 2006 Aug;94(2):141-142.
- 6. Nyboe Andersen A, Nelson SM, Fauser BC, García-Velasco JA, Klein BM, Arce JC; ESTHER-1 study group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 2017 Feb;107(2):387-396.e4.
- 7. Bayoumi YA, Dakhly DM, Bassiouny YA, Hashish NM. Addition of growth hormone to the microflare stimulation protocol among women with poor ovarian response. Int J Gynaecol Obstet 2015 Dec;131(3):305-308.
- 8. Cakmak H, Tran ND, Zamah AM, Cedars MI, Rosen MP. A novel “delayed start” protocol with gonadotropin-releasing hormone antagonist improves outcomes in poor responders. Fertil Steril 2014 May;101(5):1308-1314.
- 9. Nardo LG, Bosch E, Lambalk CB, Gelbaya TA. Controlled ovarian hyperstimulation regimens: a review of the available evidence for clinical practice. Produced on behalf of the BFS Policy and Practice Committee. Hum Fertil (Camb) 2013 Sep;16(3):144-150.
- 10. Xiao J, Chang S, Chen S. The effectiveness of gonadotropin-releasing hormone antagonist in poor ovarian responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril 2013;100(6):1594-1601.
- 11. Humaidan P, Chin W, Rogoff D, D’Hooghe T, Longobardi S, Hubbard J, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod 2017;32(3):544-555.
- 12. Afshariani R, Farhadi P, Ghaffarpasand F, Roozbeh J. Effectiveness of topical curcumin for treatment of mastitis in breastfeeding women: a randomized, double-blind, placebo-controlled clinical trial. Oman Med J 2014 Sep;29(5):330-334.
- 13. Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril 2010 Feb;93(3):855-864.
- 14. De Sutter P, Dhont M. Poor response after hormonal stimulation for in vitro fertilization is not related to ovarian aging. Fertil Steril 2003 Jun;79(6):1294-1298.
- 15. Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 1996 Apr;137(4):1447-1456.
- 16. Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 1996 Mar;81(3):1038-1045.
- 17. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011 Jul;26(7):1616-1624.
- 18. La Marca A, Grisendi V, Giulini S, Sighinolfi G, Tirelli A, Argento C, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet 2015 Jun;32(6):931-937.
- 19. Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril 2001 Dec;76(6):1185-1190.
- 20. Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 2016 Apr;4:CD001750.
- 21. Siristatidis CS, Gibreel A, Basios G, Maheshwari A, Bhattacharya S. Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database Syst Rev 2015 Nov;(11):CD006919.
- 22. Madani T, Jahangiri N. Empty follicle syndrome: the possible cause of occurrence. Oman Med J 2015 Nov;30(6):417-420.
- 23. Madani T, Jahangiri N, Eftekhari-Yazdi P, Ashrafi M, Akhoond M. Is coasting valuable in all patients with any cause of infertility? Oman Med J 2016 Nov;31(6):404-408.
- 24. Polyzos NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod 2012 Dec;27(12):3481-3486.
- 25. Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod 2015 Feb;30(2):315-322.
- 26. Mak SM, Wong WY, Chung HS, Chung PW, Kong GW, Li TC, et al. Effect of mid-follicular phase recombinant LH versus urinary HCG supplementation in poor ovarian responders undergoing IVF - a prospective double-blinded randomized study. Reprod Biomed Online 2016 Mar;34(3):258-266.
- 27. Lankarani KB, Mahmoodi M, Lotfi M, Zamiri N, Heydari ST, Ghaffarpasand F, et al. Common carotid intima-media thickness in patients with non-alcoholic fatty liver disease: a population-based case-control study. Korean J Gastroenterol 2013 Dec;62(6):344-351.
- 28. Motazedian S, Ghaffarpasand F, Mojtahedi K, Asadi N. Terbutaline versus salbutamol for suppression of preterm labor: a randomized clinical trial. Ann Saudi Med 2010 Sep-Oct;30(5):370-375.
- 29. Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod 2007 Nov;22(11):2805-2813.
- 30. Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, Diedrich K, et al; European Cetrorelix Study Group. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. Hum Reprod 2000 Mar;15(3):526-531.
- 31. Fanchin R, Castelo Branco A, Kadoch IJ, Hosny G, Bagirova M, Frydman R. Premenstrual administration of gonadotropin-releasing hormone antagonist coordinates early antral follicle sizes and sets up the basis for an innovative concept of controlled ovarian hyperstimulation. Fertil Steril 2004 Jun;81(6):1554-1559.
- 32. Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum Reprod 2004 Sep;19(9):1993-2000.
- 33. Younis JS, Soltsman S, Izhaki I, Radin O, Bar-Ami S, Ben-Ami M. Early and short follicular gonadotropin-releasing hormone antagonist supplementation improves the meiotic status and competence of retrieved oocytes in in vitro fertilization-embryo transfer cycles. Fertil Steril 2010 Sep;94(4):1350-1355.