| |
Childhood Multiple Sclerosis and Related Disorders
Amna Al-Futaisi, MD, FRCPC
ABSTRACT
Multiple sclerosis (MS) in children and adolescents is increasingly recognized worldwide. Demyelinating disorders of the central nervous system may overlap considerably in their clinical presentation and radiological appearance. Therefore an exact categorization might be challenging and might not be readily attainable. Rigorous radiological and laboratory investigations and longitudinal follow up of the patient are needed in order to establish the diagnosis of a chronic demyelinating disorder especially in children. However, in the past few years many advances have been achieved in the field of pediatric MS and other white matter disease such as establishing diagnostic criteria, consensus definitions proposed for pediatric multiple sclerosis and related disorders and advances in treating such disorders.
A wealth of knowledge regarding childhood MS have been established with vast speed and greater familiarity with the diagnosis and treatment options should lead to better care of children with such disorders. This review will present the important advances in childhood multiple sclerosis and related disorders that were achieved in the last few years.
Keywords: Childhood, Multiple sclerosis, treatment, prognosis, demyelinating diseases
From the Department of Child Health, Division of Neurology, Sultan Qaboos University Hospital, Alkhod, Sultanate of Oman.
Address Correspondence and reprint request to: Amna Al-Futaisi,
Senior Consultant, Department of Child Health, Division of Neurology
Sultan Qaboos University Hospital, P.O.Box. 38, P.C.123 Al Khodh,
Sultanate of Oman.
INTRODUCTION
Pediatric inflammatory demyelinating central nervous system diseases can be divided into two categories. Monofocal and potentially monophasic diseases like optic neuritis and transverse myelitis comprise the first group and multifocal chronic diseases like relapsing neuromyelitis optica and multiple sclerosis comprise the second.1
A diagnosis of multiple sclerosis is based on showing disease dissemination in space and time and excluding other neurological disorders that can clinically and radiologically mimic multiple sclerosis.2, 3
Multiple sclerosis (MS) in children and adolescents is increasingly recognized worldwide. The disorder presents almost exclusively as a relapsing-remitting disease in children, and most recover from the initial attacks.4
Demyelinating disorders of the central nervous system may overlap considerably in their clinical presentation and radiological appearance. Therefore an exact categorization might be challenging and might not be readily attainable. Rigorous radiological and laboratory investigations and longitudinal follow up of the patient are needed in order to establish the diagnosis of a chronic demyelinating disorder especially in children. However, in the past few years many advances have been achieved in the field of pediatric MS and other white matter disease such as establishing diagnostic criteria, consensus definitions proposed for pediatric multiple sclerosis and related disorders and advances in treating such disorders.
Early delineation of the demyelination as relapsing, mono-, or multiphasic is of paramount importance for the preservation of brain function since there are different therapeutic implications.5 A wealth of knowledge regarding childhood MS have been established with vast speed and greater familiarity with the diagnosis and treatment options should lead to better care of children with such disorders. This review will present the important advances in childhood multiple sclerosis and related disorders that were achieved in the last few years.
Incidence and Prevalence
The exact incidence of pediatric MS remains unknown. Estimates are very variable, and are likely influenced by regional and ethnic differences in MS incidence as well as the source population.2,6,7 The lack of uniform and definitive criteria to define pediatric MS could account for some of the variability. Pediatric MS also continues to be a rare entity, with an estimated 2 to 5% of patients with MS experiencing their first clinical symptoms before age 16.8 The National MS Society (NMSS) estimates that there are 8,000 to 10,000 children and adolescents with MS in the United States and another 10,000 to 15,000 children with other possibly related CNS (Central Nervous System) demyelinating disorders.9, 11
Definitions
The following definitions were agreed upon and proposed by the international multiple sclerosis study group.12
Clinically isolated syndrome (CIS), derived from the terminology used in adult patients, a CIS is defined as a first acute inflammatory demyelinating episode, either monosymptomatic or polysymptomatic, but without encephalopathy, except in cases of brainstem lesions.
Acute disseminated encephalomyelitis (ADEM), is a self-limiting, acute multifocal inflammatory demyelinating process accompanied by encephalopathy as a clinical condition ADEM can be either monophasic, recurrent (recurrence of initial symptoms in the absence of new MRI lesions) or multiphasic (new clinical ADEM event inVolving new areas of CNS).13, 14
Multiple sclerosis
A diagnosis of adult multiple sclerosis is based on showing disease dissemination in space and time and excluding other neurological disorders that can clinically and radiologically mimic multiple sclerosis. In a recent study, MRI criteria for disease dissemination in space was characterized by a good specificity (89%) when applied to patients with a subsequently confirmed diagnosis of other neurological disorders.15 Nevertheless, the common and incidental presence of non-specific white-matter abnormalities on MRI scans and the large number of disorders that have white matter abnormalities creates diagnostic difficulties that may not be reliably resolved by the application of existing MRI criteria.16
The diagnostic criteria for childhood MS are derived from those applied for adult MS patients, with the important addendum that in a child whose initial attack was diagnosed as ADEM, a second non-ADEM demyelinating event alone is not sufficient for the diagnosis of MS.12, 17
Neuromyelitis optica (NMO)
The 2006 modified NMO criteria apply also to the pediatric age group.18 Major criteria are optic neuritis and acute myelitis along with at least two of three supportive criteria (spinal MRI lesions spanning a minimum of three segments, detection of serum NMO-IgG antibody, and brain MRI not meeting diagnostic
MS criteria).19, 20
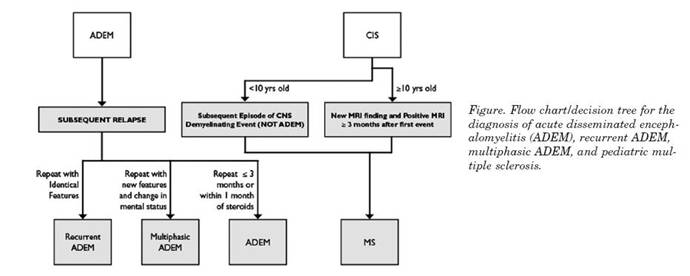
Figure 1. Flow chart/decision tree for the diagnosis of acute disseminated enceph-alomyelitis (ADEM), recurrent ADEM, multiphasic ADEM, and pediatric multiple sclerosis.
The chart below is adopted form international MS study group article and represent a useful tool for the clinician in order to tease out such conditions when in doubt.12
Clinical features
The clinical presentation of white matter disorders may be very similar and inVolve different parts of the central nervous system. A rigorous application of clinical acumen, well established diagnostic criteria and paraclinical tests is essential. Reported frequencies of visual, sensory, motor, brainstem, or cerebellar deficits in pediatric MS varied widely.9, 20, 21 Optic neuritis (both bilateral and unilateral) was identified in 0 to 50% of pediatric MS patients in the cohorts described and most of these studies reported at least 10% of patients presenting with visual changes.22 Polysymptomatic presentation was found in 10 to 67% of the patients. Predictors for a second attack includes optic neuritis, age greater than 10 years, or an MRI “suggestive of MS” with multiple well-defined periventricular or subcortical lesions.23
Diagnostic criteria for NMO have included the requirement for optic nerve and spinal cord inVolvement along with evidence of inflammation. NMO is more common in Asians, and particularly in women, but NMO is reported in all ethnic groups and has even been reported in preschool children.24, 25
Differential diagnosis
Many disorders of the central nervous system must be differentiated from pediatric multiple sclerosis. Thorough clinical history and rigorous investigations will help distinguish conditions that may resemble multiple sclerosis in their presentations. CNS infection and intracerebral malignancy must always be considered. As in adults, the diagnosis of pediatric MS can be very challenging. The younger the child and the more atypical the presenting clinical, laboratory, and neuroimaging features, may pose more difficulty in establishing the diagnosis and more care is needed in reaching a diagnosis of MS.8
When the clinician is faced with an infant or a child with acute neurologic problems and white matter abnormalities on MRI, there is a wide range of disorders to consider in the pediatric age group such as CNS Lymphoma, mitochondrial disorders, congenital leukodystrophies, CNS vasculitis and many other disorders.26-30
Although CNS lymphoma is rare in children, intracallosal inVolvement can be similar to the white matter lesions seen in MS. Primary small-vessel vasculitis of the CNS is one of the most difficult disorders to distinguish from acquired demyelination. The symptoms of macrophage-activation syndrome can initially resemble ADEM or MS. The clinical and radiographic delineation of inherited white-matter leukodystrophies are well delineated. In general, the insidiously progressive nature of inherited leukodystrophies enables them to be distinguished readily from MS, particularly because primary-progressive MS is exceptionally rare in children.
Diagnostic criteria and investigations
Diagnosis of multiple sclerosis is based on showing disease dissemination in space and time and excluding other neurological disorders that can clinically and radiologically mimic multiple sclerosis.31-34 A recommended minimum diagnostic panel for the initial inflammatory demyelinating event should define the disease burden with brain and cervical spinal cord MRI with and without gadolinium. The workup for an initial demyelinating event should also inVolve CSF studies (including cell count with differential, total protein, IgG index, evidence of oligoclonal bands, and if possible cytology). Minimum testing on a child suspected of having MS should also include complete blood count with differential, ESR, and ANA.
CSF analysis has a key role in the exclusion of acute infection and malignancy from the diagnosis of MS. The CSF white-cell count in children presenting with the first attack of MS typically ranges from 0–30 leucocytes/mm. Although cell counts of up to 60 leucocytes/mm are seen in about 8% of children, higher CSF cell counts are more characteristic of infection, vasculitis, or neuromyelitis optica. Oligoclonal bands in spinal fluid analyzed with isoelectric focusing are reported in about 90% of children with MS. However, CSF oligoclonal bands develop over the course of the disease, and not all children have positive results at first. Serum antibodies against aquaporin 4 (NMOIgG) distinguish adults with neuromyelitis optica from those with relapsing-remitting MS, with 73% sensitivity and 91% specificity.
Multimodal evoked potential testing can confirm the inVolvement of or detect clinically silent deficits in the visual evoked potential, brainstem auditory evoked potential, or somatosensory evoked potential pathways. In a study of 156 children with MS, 85 children had visual evoked potentials tested at the time of the first attack. Visual evoked potentials were abnormal in 48 children (56%), 29 of whom had no clinical evidence of optic nerve disease.34 However, investigation of brainstem auditory evoked potentials and somatosensory evoked potentials detected abnormalities not apparent in clinical examination, which is consistent with other reports.
MRI has a key role in confirming the presence of CNS lesions consistent with inflammatory demyelination and in the exclusion of other CNS disorders. Ill-defined lesions that include the deep grey nuclei in MS are more commonly seen in young children. Several studies of both children and adults have shown that MRI is not sufficient to distinguish between ADEM and MS.
Treatment
The treatment of pediatric multiple sclerosis and other related disorders are based on extrapolations from adult studies. There is lack of systematic and randomized controlled trials in this field. Despite the obvious lack of clinical trials in children it has shown that children and adolescents treated with immunomodulating drugs have a similar side effect profile as adult MS patients. The aim of drugs that modify the course of MS is to reduce the frequency of clinical relapses and to prevent the progression of disability.
Current Disease Modifying Treatments (DMTs) for MS modulate or suppress the immune system. There are four Food and Drug Administration (FDA)–approved immunomodulating agents for reducing MS relapses in adults: three preparations of interferon beta (IFNB) and glatiramer acetate (GA12).
There is also one FDA-approved immunosuppressive medication, mitoxantrone, for patients with worsening MS. Other immunosuppressive agents used empirically for patients with aggressive MS include oral azathioprine, IV immunoglobulins (IVIg), oral methotrexate, oral mycophenolate mofetil, IV cyclophosphamide, and pulses of IV methylprednisolone (MP). Natalizumab, an anti-alpha 4 integrin, was granted FDA approval for relapsing forms of MS,18-20 but safety concerns arising after three patients developed progressive multifocal leukoencephalopathy (PML) led to its temporary removal from the market.35-40 No therapeutic trial for relapses has yet been conducted in the pediatric MS population. Therefore relapse therapy is based on clinical experience, and extrapolated from studies of acute relapse management in adult MS.
Relapse therapy. As in adults, relapses associated with significant neurologic impairment warrant high-dose steroid treatment. A typical regimen used by child neurologists is IVMP 20 to 30 mg/kg/day as 1 to 2 hour infusion in the morning for 3 to 5 consecutive days. In view of potential side effects of prolonged steroid treatment in pediatric patients, a subsequent oral taper should be restricted to patients with insufficient resolution of symptoms after IVMP or those patients who experience recurrence of symptoms after IVMP discontinuation. Steroid taper should be kept as short as possible, usually not exceeding 2 to 3 weeks (e.g., prednisone 1 mg/kg/day as a single-morning dose during the first 3 days after IVMP, followed by progressive dose reduction every 2 to 3 days). IVIg may be an option for children with contraindications to corticosteroids and mild to moderate attacks, although efficacy has not been formally investigated.
Disease Modifying Treatment (DMT): Disease-modifying therapy. The International Pediatric MS Study Group agreed that immunomodulatory treatment should be started in children and adolescents with active elapsing remitting disease (defined clinically or by MRI scans) after MS is diagnosed. First-line MS therapies include approved drugs for adult MS: IFNB-1a and -1b and GA.
There have been no studies (clinical trials) documenting the efficacy of DMT in pediatric MS. Several small studies demonstrated that children treated with DMTs seem to experience some of the same adverse effects as adults. Studies documenting efficacy of therapy use in children are needed, as are large detailed safety studies. There is also a need for documentation of long-term side effects, including effects on development and psychosocial outcomes. Some children will fail treatment with the standard DMTs, necessitating use of second-line agents. Studies examining the use and effects of rescue or escalation therapies are likewise required.
Prognosis
Pediatric multiple sclerosis (MS) is often associated with a relatively high relapse rate early in the disease. However, the conversion to a secondary progressive course or long-term disability is thought to be slower in children compared to adults. A short interval (less than 1 year) between the first two demyelinating episodes, incomplete recovery after the first attack, as well as a secondary progressive disease course are unfavorable prognostic factors associated with a greater risk of developing a higher level of clinical disability over time.40,41 Increased number of attacks during the first 2 to 5 years is also associated with a higher risk of converting to secondary progressive disease.41,42
Future Directions
The use of standardized definitions for pediatric MS and related demyelinating disorders will greatly facilitate clinical care and promote a platform for future research. There is a need for collaborative international multicenter studies which can achieve the cohort size necessary to address unresolved issues given the inadequate number of patients in any given geographic area. Such information will help address several key questions such as whether CNS inflammatory demyelinating diseases have special characteristics in children as compared to adults, moreover, whether clinical presentation during the first demyelinating event can predict a mono vs multiphasic course, and finally, whether ADEM and MS represent distinctive entities. To better care for these youngsters and to advance our understanding of the biologic mechanisms underlying pediatric MS and related disorders, prospective, collaborative studies will be required.43
REFERENCES
-
Phol D, Waubant E , Banwell B et al. Treatment of pediatric multiple sclerosis and variants. NEUROLOGY 2007; 68(Suppl 2):S54–S65
-
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50:121–27.
-
Polman CH, Reingold SC, Edan G et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria“. Ann Neurol 2005; 58:840–846.
-
Banwell B, Ghezzi A, Bar-Or A et al. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007 Oct; 6:887-902.
-
Hahn JS, Pohl D, Rensel M; International Pediatric MS Study Group Differential diagnosis and evaluation in pediatric multiple sclerosis Neurology. 2007 Apr 17; 68:S13-22.
-
Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood clinical profile in 125 patients. J Pediatr 1987; 111:359–363.
-
Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood clinical features of 149 cases. Mult Scler 1997; 3:43–46.
-
Ness JM, Chabas D, Sadovnick AD; International Pediatric MS Study Group. Clinical features of children and adolescents with multiple sclerosis. Neurology. 2007 Apr 17; 68(16 Suppl 2):S37-45.
-
Boiko A, Vorobeychik G, Paty D, et al. Early onset multiple sclerosis: a longitudinal study. Neurology 2002; 59:1006–1010.
-
Mikaeloff Y, Caridade G, Assi S et al. Prognostic factors for early severity in a childhood multiple sclerosis cohort. Pediatrics 2006; 118:1133 1139.
-
Ghezzi A, Ruggieri M, Trojano, and the ITEMS Study Group. Italian studies on early-onset multiple sclerosis: the present and the future Neurol Sci 2004; 24:S346–S349.
-
Krupp LB, Banwell B, Tenembaum S, International Pediatric MS Study Group.Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007; 68(suppl 2):7– 12
-
Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children Neurology 2001; 56:1308–1312.
-
Mikaeloff Y, Adamsbaum C, Husson B, et al. MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood Brain 2004; 127:1942–1947.
-
Hahn CD, Shroff MM, Blaser SI, et al. MRI criteria for multiple sclerosis Evaluation in a pediatric cohort. Neurology 2004; 62:806–808.
-
Wingerchuk DM. Acute disseminated encephalomyelitis: distinction from multiple sclerosis and treatment issues. Adv Neurol 2006; 98:303 318.
-
Wingerchuk DM, Pittock SJ, Lennon VA, et al. Neuromyelitis optica diagnostic criteria revisited: Validation and incorporation of the NMOIgG serum autoantibody. Neurology 2005; 64:A38.
-
Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology 2003; 60:848 853.
-
Cole GF, Stuart CA. A long perspective on childhood multiple sclerosis Dev Med Child Neurol 1995; 37:661–666.
-
Gall JC, HAYLES AB, Seikert RG, et al. Multiple sclerosis in children; a clinical study of 40 cases with onset in childhood. Pediatrics 1958;21: 703–709.
-
Pohl D, Rostasy K, Treiber-Held S et al. Pediatric multiple sclerosis detection of clinically silent lesions by multimodal evoked potentials. J Pediatr 2006;149:125–127.
-
Confavreux C, Compston DA, Hommes OR, et al. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 1992;55:671–676.
-
Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53:1107–1114.
-
Bencherif MZ, Karib H, Tachfouti S, et al. Devic’s neuromyelitis optica A childhood case and review of the literature. J Fr Ophtalmol 2000; 23:488–490.
-
Ruggieri M, Iannetti P, Polizzi A, Pavone L, Grimaldi LM. Multiple sclerosis in children under 10 years of age. Neurol Sci 2004;25(suppl4) S326–335.
-
Poser CM, Goutie`res F, Carpentier MA, Aicardi J. Schilder’s myelinoclastic diffuse sclerosis. Pediatrics 1986; 77:107–112.
-
Kepes JJ. Large focal tumor-like demyelinating lesions of the brain intermediate entity between MS and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol 1993;33:18 27.
-
Tenembaum S, Galicchio S, Gran˜ana N, et al. Demyelinating encephalopathies with large focal lesions: diagnostic clues. Brain Dev 1998;20:434.
-
Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotizing encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry 1995; 58:555–561.
-
Reiber H, Peters JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001;184:101–122.
-
Ozturk A, Gurses C, Baykan B, Gokyigit A, Eraksoy M. Subacute sclerosing panencephalitis: clinical and magnetic resonance imaging evaluation of 36 patients. J Child Neurol 2002; 17:25–29.
-
Conrad AJ, Chiang EY, Andeen LE, et al. Quantitation of intrathecal measles virus IgG antibody synthesis rate: subacute sclerosing panencephalitis and multiple sclerosis. J Neuroimmunol 1994; 54:99 108.
-
Hanefeld F. Multiple sclerosis in childhood. Curr Opin Neurol Neurosurg 1992; 5:359–363.
-
Waubant E, Hieptas J, Stewart T, et al. Interferon beta-1a in children with multiple sclerosis is well tolerated. Neuropediatrics 2001; 32:211 213.
-
Banwell B. Treatment of children and adolescents with multiple sclerosis. Exp Rev Neurother 2005; 5:391–401.
-
Krupp LB, Macallister WS. Treatment of pediatric multiple sclerosis Curr Treat Options Neurol 2005; 7:191–199.
-
Pohl D, Rostasy K, Ga¨rtner J, Hanefeld F. Treatment of early onset multiple sclerosis with subcutaneous interferon beta-1a. Neurology 2005; 65:888–890.
-
Banwell B, Reder AT, Krupp L, et al. Safety and tolerability of interferon beta-1b in pediatric multiple sclerosis. Neurology 2006; 66:472–476.
-
Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology 2002; 59:1922–1928.
-
Ozakbas S, Idiman E, Baklan B, et al. Childhood and juvenile onset multiple sclerosis: clinical and paraclinical features. Brain Dev 2003; 25:233–236.
-
Mikaeloff Y, Suissa S, Vallee L, et al. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr 2004; 144:246–252.
-
Shiraishi K, Higuchi Y, Ozawa K, et al. Clinical course and prognosis of 27 patients with childhood onset multiple sclerosis in Japan. Brain Dev 2005; 27:224–227.
-
Belman AL, Chitnis T; Renoux C et al. Challenges in the classification of pediatric multiple sclerosis and future directions. NEUROLOGY 2007;68(Suppl 2):S70–S74.
|
|