|
Introduction
Cutaneous Mycosis Fungoides (MF), the subtype of cutaneous T-cell Lymphoma is a clinically and pathologically distinct form of peripheral extra nodal non-Hodgkin’s T-cell lymphoma. It was first described by the French dermatologist, Alibert in 1806.1 In the new WHO classification, early MF is reserved for those indolent cases having classical features and in which there is a progression from patches to plaques to tumors. The etiology of MF is still unclear,2 but current hypotheses include genetic and epigenetic abnormalities.3 Histocompatibility antigen association have also been noted with MF, suggesting a possible genetic predisposition.4,5 An infectious etiology has been suggested but no infectious agent has been isolated to date. The peak age at presentation is in excess of 55 to 60 years, with a male to female ratio of 2:1.6
Histologically, MF is characterized by the presence of a variable dense infiltrate of atypical lymphoid cells in the papillary dermis with extension of these cells into the epidermis (Epidermotropism). When making a diagnosis of MF during the early/patch stages of the disease, the architectural abnormalities are more important than the cytological atypia of the lymphocytes, which is usually mild. It is for this reason that the manifestations of early MF may mimic and overlap both clinically and histologically with those of other benign inflammatory dermatoses.7
The purpose of the present study was to assess the frequency and significance of the different pathological parameters that are seen in Saudi patients in early MF. These parameters when identified can also be used to differentiate between early MF and various inflammatory dermatoses.
Methods
Sixty-six skin biopsies generated from 58 patients who had either clinically suspicious MF lesions or were clinically diagnosed as being in the early patch stage of MF were reviewed. The cases were retrieved from the archives of the department of Pathology at King Khalid University Hospital, Riyadh, and included all cases between the years 2002 and 2006. The final diagnosis of MF was confirmed in all the cases either by correlation with the clinical features or by the presence of histological findings regarded to be characteristics of MF. Punch biopsies were taken at the onset of the disease or from recurrent patches, after stopping all topical treatment including PUVA therapy at least 2-4 weeks prior to the biopsy. The biopsies were fixed in buffered formalin, routinely processed and stained with Hematoxylin and Eosin stains. A person not taking part in the histological evaluation randomly numbered the slides from 1-66. Each slide was then independently reviewed by the two participating histopathologists and one dermatopathologist. A total of 16 histological criteria were assessed and each criterion was graded semi-quantitatively (0, 1+, 2+, 3+), (Table 1). For any of the criteria to be considered as present, it was mandatory to be graded as 1+ or above.
Epidermotropism was graded according to the presence of lymphocytes deposited as solitary units within or near the basal cell layer of the epidermis (Fig. 1). Spongiosis (intercellular edema) was defined as the presence of widened and edematous intercellular spaces but without the formation of Langerhan's cell containing micro vesicles. Papillary dermal fibrosis was defined as the presence of increased "wire like" dermal collagen. Pautrier’s micro abscesses were defined as a collection of four or more lymphoid cells in the epidermis with no significant cytopathic changes in the surrounding keratinocytes (Fig. 2). "Basal alignment of neoplastic lymphocytes" was defined as the presence of single lymphoid cells, closely apposed to the basal layer of the epidermis.8,9
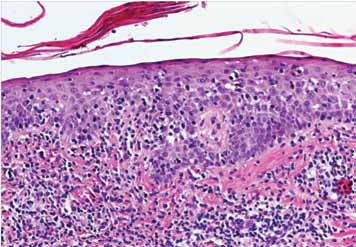
Figure 1: Infiltrate of atypical lymphocytes along slightly expanded papillary dermis, extending upwards and colonizing in the epidermis (Epidermotropism).
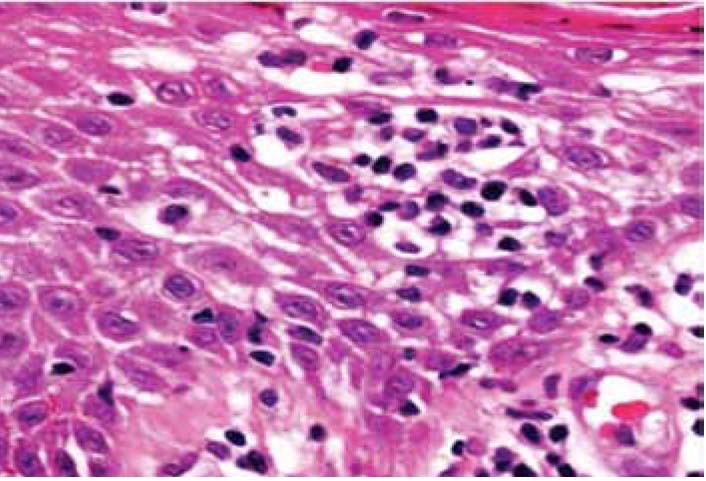
Figure 2: Collection of lymphoid cells in the epidermis with no significant cytopathic changes in the surrounding keratinocytes (Pautrier’s micro abscess).
The data were analyzed using statistical software STATA version 10 for windows. The univariate analysis was performed on each morphological criterion by the chi-square or Fisher’s exact test. A p-value of less than 0.05was considered significant. For each histological test, sensitivity, specificity, predictive values, and likelihood ratios were calculated with the groups with confirmed MF and negatives using the "diagt" STATA procedure. Logistic Regression analysis was used to study the association between mycosis fungoides and selected histological parameters. Sensitivity is the likelihood that a diseased patient has a positive test, while specificity is the likelihood that a healthy patient has negative test. The predictive value of test positive is the likelihood that a person who tests positive has the disease, while the predictive value of test negative is the likelihood that a person who tests negative does not have the disease. The likelihood ratio (LR) compares the probability of obtaining a correct test result with that of containing an incorrect test result for positive and negative test results, respectively. LR positive = (Sensitivity/1-specificity); LR negative=(1-sensitivity)/specificity. LR above or below 1 increases or decreases the likelihood of the disease, respectively.
Results
Sixty-six slides generated from 58 patients were received. In six patients, a repeat biopsy and in one patient, three repeat biopsies were required to reach the final diagnosis. The age of the patient in the different groups was between 5-74 years; while the male to female ratio was 1.9:1 (38:20). Five of the studied patients who were diagnosed as being in the patch stage were children less than ten years of age.
The results of the histological evaluation of sixty-six slides are summarized in Table 2, along with the specificity and sensitivity, positive predictive value, negative predictive value and likelihood ratio calculated for each recorded feature. Of these, 38 cases were clinically and histologically confirmed for patch stage MF. Among the remaining 28 cases which were not clinically confirmed, twenty were suspicious of MF and were advised careful follow up. Eight cases were negative for MF on histology. These negative cases were diagnosed based on clinic-pathological correlation as drug reaction (three cases), psoriasiform dermatitis (two cases), pruritic dermatosis (two cases) and pityriasis alba (one case).
The histological criteria that were useful for discriminating inflammatory and patch stage lesions of MF as documented by the univariate and multivariate statistical analysis are summarized in Table 2. The statistically significant criteria (p<0.05) used for differentiating cases which were negative for MF from the confirmed patch stage cases of MF included dermal fibrosis, hyperconvoluted epidermal and dermal lymphocytes, grandiosity sign, Basal alignment of neoplastic lymphocytes and Pautrier’s micro abscesses. These findings emphasize the importance of both cytological and architectural features in the diagnosis of MF.
Table 1: Grading of histologic parameters during the assessment of suspected Mycosis Fungoides.
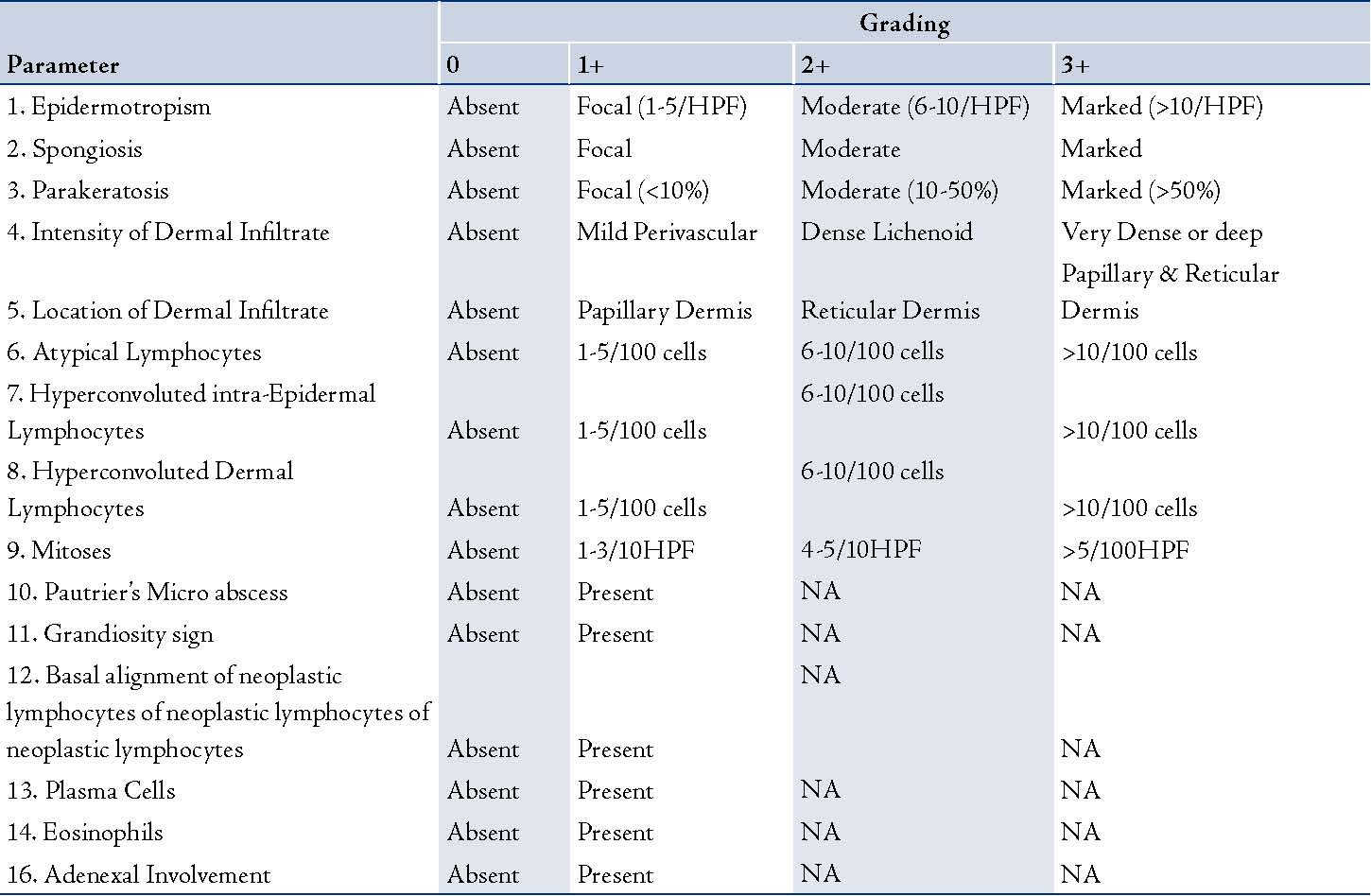
HPF- High power field; NA- Not Applicable
Table 2: Histological features of Mycosis Fungoides (MF; n=38), suspicious for mycosis Fungoides (n=20) and non mycosis Fungoides (n=8) with sensitivity, specificity, positive predictive value, negative predictive value and likelihood ratio.
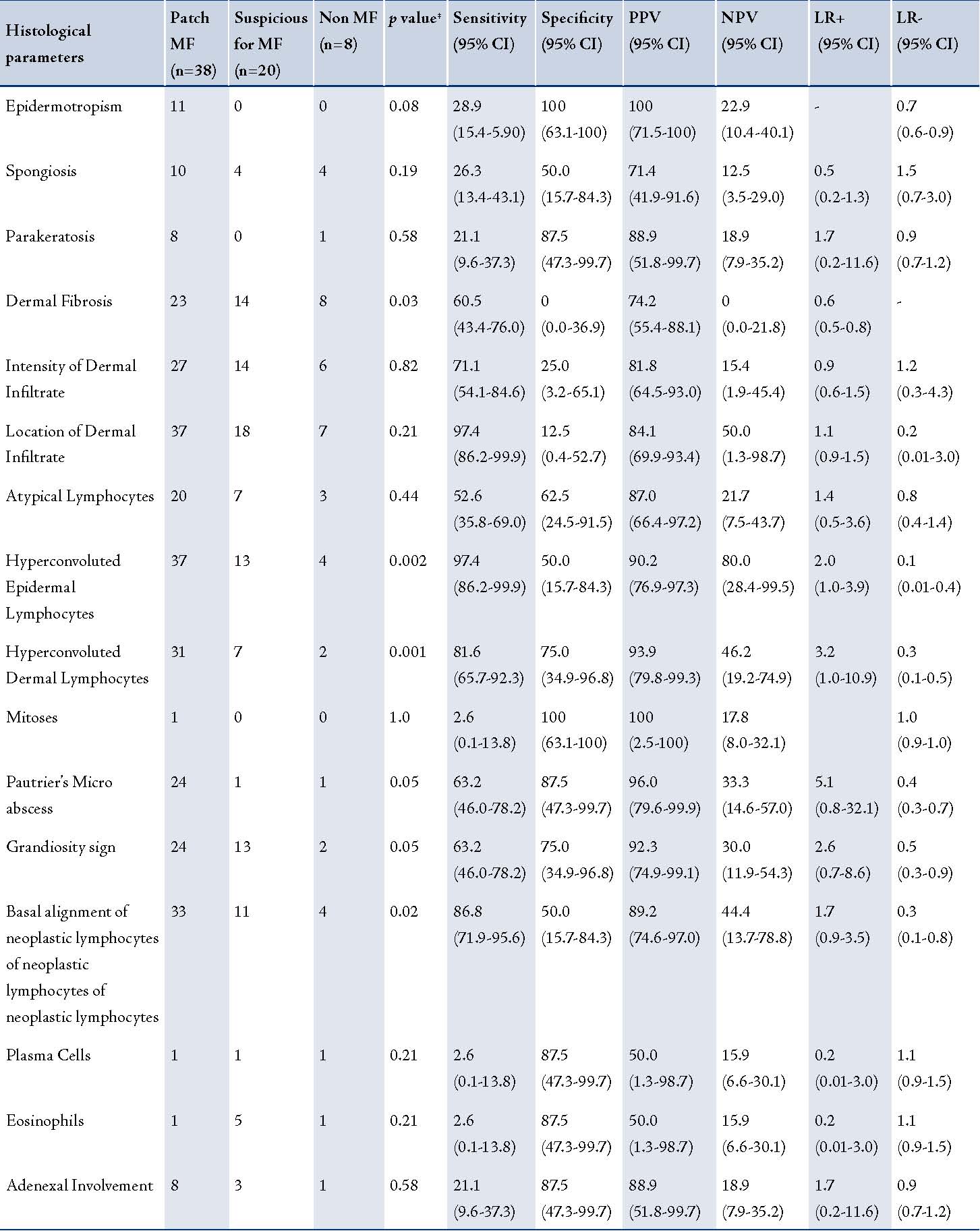
All association and diagnostic tests were done using patch MF and non-MF.
PPV- positive predictive value; NPV-negative predictive value; LR-likelihood ratio; ‡ p-values were obtained using chi-square.
The criteria that were moderately sensitive and highly specific for MF included Pautrier’s micro abscesses and grandiosity sign. Basal alignment of neoplastic lymphocytes also showed a high degree of sensitivity with moderate specificity. Pautrier’s micro abscess and basal alignment were the two histological parameters significantly associated with mycosis fungoides in both unadjusted and adjusted logistic regression analysis. There were higher odds of MF in these two histological parameters with positives compared to the negatives.
The parakeratosis, epidermotropism, plasma cells, eosinophils, adenexal involvement and mitotic rate were highly specific but showed very low sensitivity. A moderate or high degree of sensitivity was however achieved by determining the intensity and location of the dermal infiltrate with low specificity. The presence of hyperconvoluted lymphocytes in the epidermis and dermis showed that the most significant discrimination between cases and non-cases of MF using various diagnostic statistical tests. (Table 2)
Discussion
MF is a difficult diagnosis to make in its initial stages for both the clinicians and the pathologists. Immunophenotyping and T-cell receptor gene rearrangement studies by polymerase chain reaction have been utilized as adjuvant techniques to provide additional useful information in difficult cases; however, the immunophenotypic-abbrations seen in advanced stages of MF may not be found in the patch stage.10 The histopathological examination of skin biopsies is thus considered very important and the single most reliable laboratory tool in addition to clinical history that helps the clinician to establish the diagnosis of MF. Several follow up skin biopsies are usually required to achieve this goal.11 The histological diagnosis of fully developed MF is generally easy because of the presence of morphological findings typical for this disorder. Histopathological diagnosis of early MF is however both difficult and challenging. This is because early lesions may show minimal changes that are easily confused with inflammatory dermatoses including atypical presentation of common conditions such as eczema and psoriasis.12 Although there have been many studies on early MF, there is a lack of consensus on what constitutes a specific histological criterion leading to the diagnosis of MF among the dermatopathologists.13,14
In this study, we did not focus on the establishment of new histopathological criteria for the diagnosis of early MF, but merely recorded the patterns observed in Saudi patients who had MF confirmed by clinico-pathologic correlation. The findings were compared with those published in international medical literature and a systematic evaluation of many previously reported criteria deemed to be useful in distinguishing MF from its inflammatory simulators was performed.15 By strictly defining the criteria before initiating a comparison, the inter and intra observer disagreements often encountered during histological evaluations were minimized.
Historically, many investigators have proposed various subtle histological findings as clues to the correct diagnosis of early MF.13,15,16 However, in the early stages; clinical pictures may be more suggestive than the histopathological features. In addition, more reliance has to be placed on clinico-pathological correlation.17 On light microscopy, parameters strongly suggestive of the diagnosis of MF include; atypical intra-epidermal lymphocytes which are surrounded by halos, Pautrier’s micro abscesses, disproportionate epidermotropism, epidermal lymphocytes larger than dermal lymphocytes, hyperconvoluted intraepidermal lymphocytes aligned within the basal layer and the papillary dermal fibrosis within a lichenoid infiltrate.13,15,16,18
Albert and Amy observed that cytological lymphocytic atypia within the dermis or epidermis does not appear to be useful as the sole criteria for arriving at a diagnosis of MF.7 This is despite the fact that the increased number of mononuclear cells (not necessary atypical) distributed singly or in small clusters within the epidermis is an extremely important parameter.8,19,20 Ackerman reported four parameters as being the diagnostic criteria of early MF.21 These include exocytosis with paucity of spongiosis, solitary lymphocytes lined up along the basal layer, epidermal lymphocytes larger than dermal lymphocytes and papillary dermal fibrosis within a lichenoid infiltrate. While Smoller concluded that haloed lymphocytes proved to be the most robust discriminator of MF from non-MF on multivariate analysis studies.15 Shapiro and Pinto analyzed various morphological features and concluded that early MF produces practically all the parameters used for diagnosing inflammatory skin diseases.16 However, they suggested common clues to the diagnosis of early MF which included epidermotropism with little spongiosis, lymphocytes lined up along the basal layer, hyperconvoluted lymphocytes and broad areas of slight hyper-orthokeratosis that is compact or laminated, with subtle interspersed parakeratosis. The epidermotropism is considered to be the hallmark of MF and has been reported in 75-100% of cases.9,13,15,18,22 In the current study, epidermotropism was found in 29% of MF patients and absent in suspicious cases and inflammatory mimickers. It is therefore a highly specific but not a sensitive parameter. We found that Basal alignment of neoplastic lymphocytes is a significant differentiating factor between MF positive and non-MF cases. This feature was present in 86.8% of the studied MF cases and in 55% of the inflammatory lesions. This finding is in agreement with findings reported in the literature.9,13,15,18
Pautrier’s micro abscesses were detected in patches of MF and very occasionally in its inflammatory simulators (63.2% sensitivity and 87.5% specificity), proving it to be a pertinent discriminator on statistical analysis. In the literature, this feature is commonly described as specific by many investigators.18,20,23
In agreement with previous studies; spongiosis, parakeratosis intensity and location of dermal infiltrate were all seen in variable numbers, both in the patch stage of MF and in inflammatory skin lesions.13,15 This indicates that the presence of these features is not very supportive for the diagnosis of MF. Plasma cells and eosinophills were uncommon in the dermal infiltrate during the patch stage of MF. Eosinophills were however, detected more frequently in inflammatory dermatoses. The current study showed that hyper-convoluted epidermal and dermal lymphocytes proved to be a discriminatory factor with high sensitivity and specificity. This was further supported by higher predictive values and the high and low likelihood ratio values of positive and negative, respectively. This finding is in agreement with the EORTC classification,13 which concluded that the presence of single or clusters of medium to large sized cerebriform cells in the epidermis or dermis is the single most useful histologic criterion for establishing a diagnosis of early MF.
Conclusion
Overall, there is no doubt that the histopathologic differentiation between early MF lesions and non-MF cases is one of the most challenging areas in skin pathology. To rely on any single histopathologic feature only in the diagnosis of early MF is incorrect. This study proved that the presence of hyperconvoluted lymphocytes in the epidermis and dermis proved to be a highly reliable feature, if used in conjunction with epidermotropism, Pautrier’s micro abscess, grandiosity sign and lymphocytic Basal alignment of neoplastic lymphocytes. Thus, sequential and multiple site biopsies as well as meticulous follow-up of younger patients in whom the histopathological diagnosis is in doubt are also recommended. This constitutes a better approach instead of rendering a hasty diagnosis in such cases.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
1. Alibert JL. Description des Maladies de la Peau: Observees l'Hospital St. Louis et Exposition des Meilleurs Methods Suiview pour leur Traitement. Paris. In: Barrois l'aine et Fils, 1806.
2. Whittaker S. Biological insights into the pathogenesis of cutaneous T-cell lymphomas (CTCL). Semin Oncol 2006 Feb;33(1)(Suppl 3):S3-S6.
3. Shin J, Monti S, Aires DJ, Duvic M, Golub T, Jones DA, et al. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood 2007 Oct;110(8):3015-3027.
4. Rosen ST, Radvany R, Roenigk H Jr, Terasaki PI, Bunn PA Jr. Human leukocyte antigens in cutaneous T cell lymphoma. J Am Acad Dermatol 1985 Mar;12(3):531-534.
5. Hodak E, Klein T, Gabay B, Ben-Amitai D, Bergman R, Gdalevich M, et al. Familial mycosis fungoides: report of 6 kindreds and a study of the HLA system. J Am Acad Dermatol 2005 Mar;52(3 Pt 1):393-402.
6. Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol 2007 Jul;143(7):854-859.
7. Yeh YA, Hudson AR, Prieto VG, Shea CR, Smoller BR. Reassessment of lymphocytic atypia in the diagnosis of mycosis fungoides. Mod Pathol 2001 Apr;14(4):285-288.
8. Glusac EJ. Criterion by criterion, mycosis fungoides. Am J Dermatopathol 2003 Jun;25(3):264-269.
9. Inchara YK, Rajalakshmi T. Early mycosis fungoides vs. inflammatory mimics: how reliable is histology? Indian J Dermatol Venereol Leprol 2008 Sep-Oct;74(5):462-466.
10. Bergman R, Faclieru D, Sahar D, Sander CA, Kerner H, Ben-Aryeh Y, et al. Immunophenotyping and T-cell receptor gamma gene rearrangement analysis as an adjunct to the histopathologic diagnosis of mycosis fungoides. J Am Acad Dermatol 1998 Oct;39(4 Pt 1):554-559.
11. Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, Stevens S, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol 2005 Dec;53(6):1053-1063.
12. Nashan D, Faulhaber D, Ständer S, Luger TA, Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol 2007 Jan;156(1):1-10.
13. Santucci M, Biggeri A, Feller AC, Massi D, Burg G; European Organization for Research and Treatment of Cancer. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. Am J Surg Pathol 2000 Jan;24(1):40-50.
14. Parker SR, Bethaney JV. Cutaneous T cell lymphoma-mycosis fungoides and Sezary syndrome: an update. G Ital Dermatol Venereol 2009 Aug;144(4):467-485. PubMed.
15. Smoller BR, Bishop K, Glusac E, Kim YH, Hendrickson M. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol 1995Dec;19(12):1423-1430.
16. Shapiro PE, Pinto FJ. The histologic spectrum of mycosis fungoides/Sézary syndrome (cutaneous T-cell lymphoma). A review of 222 biopsies, including newly described patterns and the earliest pathologic changes. Am J Surg Pathol 1994 Jul;18(7):645-667.
17. Caro WA. Biopsy in suspected malignant lymphoma of the skin. Cutis 1978 Feb;21(2):197-201.
18. Naraghi ZS, Seirafi H, Valikhani M, Farnaghi F, Kavusi S, Dowlati Y. Assessment of histologic criteria in the diagnosis of mycosis fungoides. Int J Dermatol 2003Jan;42(1):45-52.
19. Sanchez JL, Ackerman AB. The patch stage of mycosis fungoides. Criteria for histologic diagnosis. Am J Dermatopathol 1979;1(1):5-26.
20. Nickoloff BJ. Light-microscopic assessment of 100 patients with patch/plaque-stage mycosis fungoides. Am J Dermatopathol 1988 Dec;10(6):469-477.
21. Ackerman AB. An algorithmic method for histologic diagnosis of inflammatory and neoplastic skin diseases by analysis of their patterns. Am J Dermatopathol 1985Apr;7(2):105-107.
22. Massone C, Kodama K, Kerl H, Cerroni L. Histopathologic features of early (patch) lesions of mycosis fungoides: a morphologic study on 745 biopsy specimens from 427 patients. Am J Surg Pathol 2005 Apr;29(4):550-560.
23. Cho-Vega JH, Tschen JA, Duvic M, Vega F. Early-stage mycosis fungoides variants: case-based review. Ann Diagn Pathol 2010 Oct;14(5):369-385.
|