Intraosseous hemangioma (IH) is not a frequently-encountered entity and usually affects the vertebral body and skull vault. When found in these bones, the diagnosis is not difficult due to its typical appearance. It is challenging to make a firm diagnosis on imaging when seen in unusual locations such as a long bone. We present such a case of histologically-proven IH affecting the proximal ulnar metaphysis and describe its imaging findings. A literature search revealed only one other reported case of proximal ulna IH to date.1
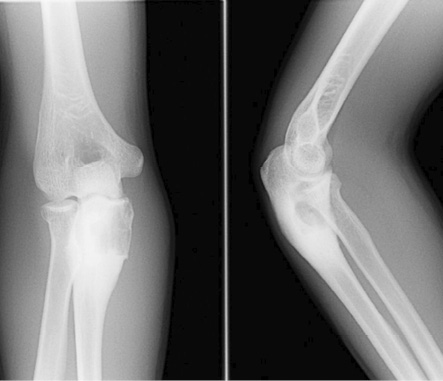
Figure 1: Frontal and lateral radiograph of elbow shows an expansile osteolytic lesion with a narrow zone of transition and surrounding sclerosis in the proximal metaphysis of the right ulna.
Case Report
A 19-year-old boy presented with right elbow pain of one-week duration. The pain persisted despite conservative therapy consisting of analgesics and non-steroidal anti-inflammatory drugs. There was no history of trauma. No soft tissue swelling or tenderness was noted on physical examination. The elbow had a normal full range of movement including supination and pronation. The elbow radiograph showed an expansile osteolytic lesion with surrounding sclerosis and a narrow zone of transition in the proximal metaphysis of the right ulna. There was no cortical erosion, periosteal reaction, or pathological fracture [Figure 1]. Further evaluation with pre- and post-contrast magnetic resonance imaging (MRI) showed a 2.1 × 1.6 × 3.1 cm lesion in the proximal metaphysis of the ulna, which was hyperintense on T2-weighted imaging (T2WI) and isointense to skeletal muscle on T1-weighted imaging (T1WI) [Figure 2a and b] and showed internal trabeculae. Mild rim enhancement was present on the post-contrast fat-saturated (FS) T1WI [Figure 2c]. Computed tomography (CT) showed a lobulated osteolytic lesion with well-defined margins, internal trabeculae, and surrounding sclerosis [Figure 3a]. Technetium-99m-methylene diphosphonate (99mTc-MDP) bone scan revealed a focus of intense tracer uptake in the proximal ulna [Figure 3b]. No abnormal tracer uptake was detected elsewhere. Based on the patient’s age and imaging features, differential diagnosis raised were those of a non-aggressive bone tumor, such as fibrous dysplasia, giant cell tumor, and aneurysmal bone cyst. The patient underwent open surgery involving open biopsy and curettage, followed by bone grafting. Histopathology revealed proliferative, branching blood vessels within the tissue lined by endothelial cells with solid nests of bland epitheliod endothelial cells [Figure 4a and b]. CD31 immunomarker showed endothelia in the proliferative vessels with patent lumina [Figure 4c]. The final diagnosis was IH. The patient had an uneventful postoperative course. On six-month follow-up, the patient was asymptomatic and the osteolytic lesion showed interval healing with areas of sclerosis [Figure 5].
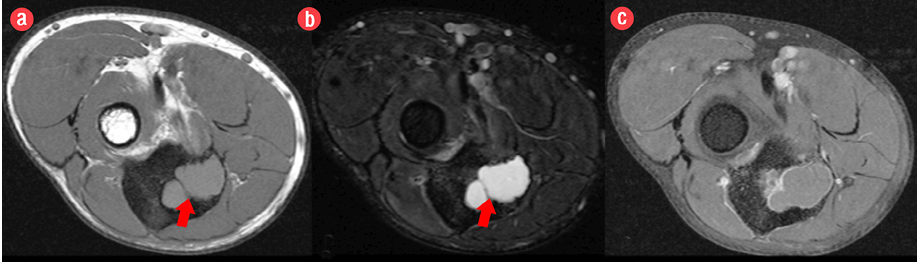
Figure 2: Pre- and post-contrast axial magnetic resonance images revealed a lesion in the proximal metaphysis of the ulna, which was (a) isointense on T1-weighted imaging and (b) hyperintense on fat-saturated T2-weighted imaging with internal trabeculae. (c) Post-contrast fat-saturated T1-weighted imaging demonstrated mild rim enhancement.
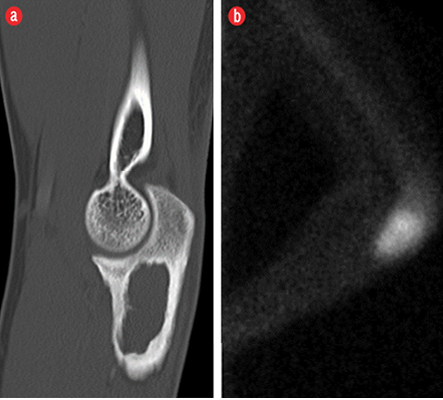
Figure 3: (a) Sagittal reconstructed computed tomography image showed a lobulated osteolytic lesion with well-defined margins, few internal trabeculae, and surrounding sclerosis in the proximal metaphysis of the ulna. (b) Technetium 99m-methylene diphosphonate bone scan showed a focus of intense tracer uptake in the proximal ulna.
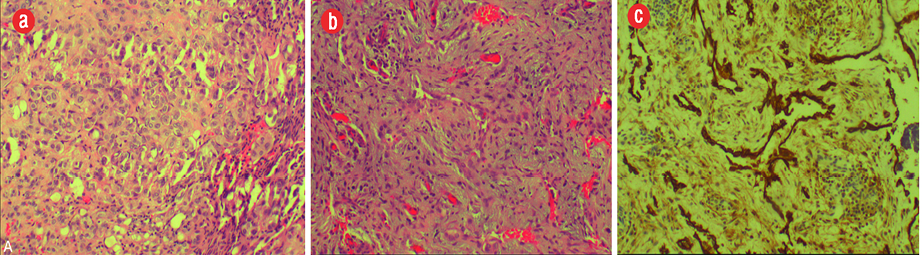
Figure 4: Histopathology of the excised specimen showed (a) branching proliferative blood vessels lined by bland endothelia and separated by inflamed edematous fibrous tissue. (b) It also showed solid nests of bland epithelioid endothelial cells in keeping with epithelioid hemangioma component. (c) The CD31 immunomarker highlighted endothelia in the proliferative vessels with patent lumina, magnification = 200 ×.
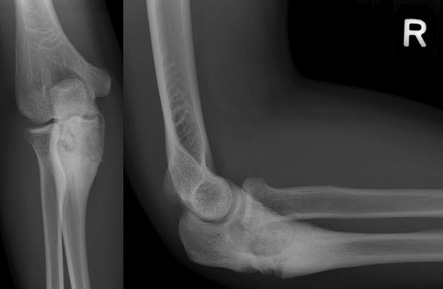
Figure 5: Follow-up frontal and lateral radiographs of the right elbow after six-months revealed areas of sclerosis in keeping with expected healing.
Discussion
IH is rare and accounts for < 1% of all bone neoplasms. It can affect any age group, particularly those 40–50 years old.2,3 IH commonly affects the spine and skull accounting for more than 75% of cases. Long bone involvement is extremely rare.1,2 Among the long bones, IH usually affects bones of the lower extremity (73%) especially the femur and tibia and, less commonly, the upper extremity bone.3 Furthermore, ulnar involvement is rare, being seen in < 1% of all bone hemangiomas.1,4 Literature review revealed only one reported case of solitary IH in the proximal ulna.1 IH affecting long tubular bones can be medullary, periosteal, and intracortical. Medullary IH commonly affects the diaphysis of a long bone.5 IH involving the appendicular skeleton are often symptomatic, unlike those of the axial skeleton, which are generally asymptomatic.6
The radiological appearances of IH in the appendicular skeleton is highly variable and non-specific, and thus it is often difficult to diagnose these lesions preoperatively. Diverse internal matrix found in IH such as fat, vessels, smooth muscle, fibrous tissue, and clotted blood may account for this variable radiological appearance.3,5 On radiographs, medullary IH found in the metaphysis of long bones usually present as an osteolytic lesion with internal trabeculae, giving honeycomb or soap-bubble appearance. This results from expansive proliferation of engorged vessels and thickened remodeled bone trabeculae. The lesion may have spiculated periosteal reaction. In rare cases, medullary hemangiomas may appear completely lucent on radiographs as in our case.1 On CT, long bone IH corresponds with its appearance on radiographs and are seen as an osteolytic lesion with internal trabeculae.5 These CT findings were similar to our case. In some cases, the thickened vertical trabeculae give a ‘polka dot’ appearance as seen in the spinal variety.3,5 Long bone IH have a variable appearance on MRI and may show low, intermediate, or high signal intensity on T1WI. The hyperintense signal sometimes seen on T1WI may be due to the fat content within the lesion. On T2WI, IH typically appears hyperintense due to the fluid content of the tumor vessels. T1 and T2WI characteristically may show hypointense internal trabeculae within long bone IH as in our case.3 On post-contrast FST1WI images, long bone IH may show marked to minimal or no enhancement.1,6 The MRI findings of long bone IH are variable and usually non-diagnostic. The role of MRI may be restricted to defining the nature and extent of the lesion.1
On 99mTc-MDP bone scans, long bone IH usually shows normal uptake. However, in some cases, they may demonstrate decreased or increased activity.7 This may be explained by the variable internal vascular nature of the lesion. In our case, the lesion showed increased uptake on bone scan.
Small asymptomatic long bone IH usually require no surgical treatment. Curettage and bone grafting are usually reserved for large, symptomatic lesions or lesions with pathologic fracture.1 In our case, the lesion was symptomatic, with a risk of pathological fracture and curettage with bone grafting was performed.
Conclusion
Unlike axial bone hemangioma, imaging diagnosis of an appendicular bone hemangioma is challenging, due to its rarity and diverse imaging appearances. Histopathological examination usually confirms the diagnosis. Hemangioma involving the proximal ulna is extremely rare; however, a radiologist should be aware and consider this entity in the differential diagnosis of a medullary based, expansile, osteolytic lesion with soap-bubble appearance affecting a long tubular bone, particularly on CT and MRI.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Matsumoto K, Ishizawa M, Okabe H, Taniguchi I. Hemangioma of bone arising in the ulna: imaging findings with emphasis on MR. Skeletal Radiol 2000 Apr;29(4):231-234.
- 2. Cha JG, Yoo JH, Kim HK, Park JM, Paik SH, Park SJ. PET/CT and MRI of intra-osseous haemangioma of the tibia. Br J Radiol 2012 Apr;85(1012):e94-e98.
- 3. Rigopoulou A, Saifuddin A. Intraosseous hemangioma of the appendicular skeleton: imaging features of 15 cases, and a review of the literature. Skeletal Radiol 2012 Dec;41(12):1525-1536.
- 4. Mirra JM. Vascular tumors. In: Mirra JM, Picci P, Gold RH (eds) Bone tumors. Clinical, radiographic, and pathologic correlations. Philadelphia: Lea & Febiger, 1989: 1335-1478.
- 5. Xia Z, Sittampalam K, Howe TS, Lo NN. Successful treatment of solitary intraosseous haemangioma of the femoral neck. Singapore Med J 2015 Apr;56(4):e65-e70.
- 6. Chawla A, Singrakhia M, Maheshwari M, Modi N, Parmar H. Intraosseous haemangioma of the proximal femur: imaging findings. Br J Radiol 2006 Aug;79(944):e64-e66.
- 7. Ching BC, Wong JS, Tan MH, Jara-Lazaro AR. The many faces of intraosseous haemangioma: a diagnostic headache. Singapore Med J 2009 May;50(5):e195-e198.