Sleep is a biological phenomenon characterized by reversible loss of awareness and lack of response to external stimuli.1 Sleep is divided into two phases: non-rapid eye movement (NREM) and rapid eye movement (REM).2,3 According to the American Academy of Sleep Medicine (2015) guidelines,2 the NREM phase is divided into three stages instead of four as was previously classified,3 depending on the depth of sleep. Identified by electroencephalography (EEG), N1 shows drop out of alpha activity and appearance of theta waves, N2 is characterized by sleep spindles and K-complexes, while N3 predominately shows higher amplitude delta waves. REM sleep is characterized by intermixed background activity, random eye movements, and sawtooth waves.2,3
Sleep is known to provoke seizures and facilitates the appearance of interictal epileptiform discharges4−7 in both generalized or partial onset epilepsy particularly during NREM sleep.8,9 This effect of sleep was demonstrated in sleep studies that involved long periods of monitoring that could exceed 72 hours, and hence, increases the chances to record seizure activity.10−14
A standard routine EEG commonly includes a short period of early preliminary sleep called a nap with the aim of showing interictal abnormalities or even to provoke seizures.15 It consists of the very early stages of sleep that shows the transition from wakefulness to early sleep phases which can be either N1, or N1 and N2.15 It is identified as an important component of the EEG test and included in the guidelines of prominent Clinical Neurophysiological Societies, like the American, British, and European Electroencephalographic Societies.16–18
This practice is also followed in our EEG lab at Sultan Qaboos University Hospital (SQUH), Muscat, Oman, and we incorporate a nap sleep in the setting of a routine EEG test.19 A recent review of the literature showed no current reports on the usefulness of this practice and its contribution to EEG diagnostic outcomes. The role of nap sleeping compared to sleep EEGs achieving full slow wave sleep in showing interictal abnormalities and seizures remains to be defined. We examined the contribution of the nap sleep to the final EEG report by examining its sensitivity in showing interictal abnormalities or by provoking seizures.
Methods
This audit study reviewed all the available EEGs data performed in the Department of Clinical Physiology at SQUH from July 2006 to December 2007 and from January 2009 to December 2010 (total 42 months). The year 2008 was not included because of missing data. All the included EEGs were from participants older than 13-years and were performed for possible epilepsy, blackouts, headache, head trauma, and other non-specified attacks. None of the EEGs were for severely ill or comatose patients from the intensive care unit.
EEG recording was digital and we used Grass Telefactor; Comet series’ model: CMXLE-230 (Grass Technologies, Warwick, Rhode Island, USA) machine. The recording used Ag-AgCl disc electrodes, followed the 10/20 international montage, and was carried out with the patient in a supine position for about 20 minutes and included baseline wakefulness and activation procedures such as hyperventilation and photic stimulation.19
The nap sleep was obtained if the participant spontaneously slept at any time during recording with another 10−20 minutes of recording allowed. We defined EEG abnormalities as interictal epileptiform spike and sharp waves, focal or generalized slow waves during awake state, and abnormal asymmetry of background activity.19,20 Statistical analysis was performed using Excel program (office 2013).
Results
A total of 2 547 EEGs were reviewed with the age range of 13−95 years, 76.9% were below 36 years (mean±standard deviation (SD), 28.3±15.8 years). There were 744 (29.2%) abnormal EEGs obtained. Figure 1 shows further analysis of these abnormal EEGs (n = 744). Of the abnormal EEGs (n = 744), 258 (34.7%) EEGs with nap sleep were obtained. All the abnormalities during nap sleep were either sharp or spike discharges.
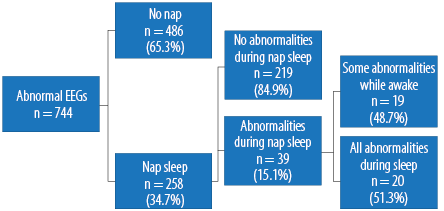
Figure 1: Flow chart of the follow-up of the abnormal EEGs (n = 744).
The majority (84.9%) of those who had nap sleep showed all their abnormal EEGs while awake. Out of the abnormal EEGs with nap sleep (n = 258), there were 39 (15.1%) that showed EEG abnormalities during nap sleep. It is thus estimated that about 15.1% of those who have nap sleep show abnormal EEGs during sleep. A 95% confidence interval (CI) for the proportion that shows abnormal EEGs during nap sleep is (0.107, 0.195). We can predict with 95% confidence that among those who have nap sleep between 10.7% and 19.5% will show abnormal EEGs during sleep. This suggests that nap sleep does not increase the chances of abnormal EEGs and does not provoke seizures. Following those 39 who showed abnormal EEGs during nap sleep, there were 19 (48.7%) abnormal EEGs during awake and nap sleep and 20 (51.3%) abnormal EEGs during nap sleep.
A 95% CI for this proportion is (0.356, 0.670). We might predict with 95% confidence that out of those who show abnormal EEGs during nap sleep, between 35.6% and 67.0%, did so only during nap sleep. This represent 7.8% of the total abnormal nap sleep EEGs (n = 258) and 2.7% of the total abnormal EEGs (n = 744) and 0.78% of the total EEGs performed.
Discussion
In our current practice at SQUH, we provide an unselected spontaneous nap sleep opportunity for all patients undergoing EEG. Slightly greater than a third (34.7%) of the EEGs were abnormal and of these only 2.7% showed epileptiform abnormalities during the period of nap sleep. Much of the literature on abnormalities seen during sleep looks at prolonged studies that in many lasted at least more than a day and on average of 3–4 days with several episodes of transition between wakefulness and deep NREM sleep, and REM and NREM sleep.10–14 Our audit study suggests that attempting to obtain this information with brief unselected nap sleep EEGs may not be the most effective strategy to follow.
A significant number of participants with abnormal EEGs were unable to spontaneously sleep (65%), which might have influenced the outcome. However, this reflects the actual environment in a busy department. Factors like tranquility, test interruption, comfort, and technical assistance should be addressed and rectified.
A number of limitations were identified in this study that includes a heterogeneous population
and skewed distribution towards a younger population. This could be because the study was retrospective and reported from a single EEG laboratory. Cautious future prospective studies should be considered.
Conclusion
This audit study addressed the additional value of nap sleep to EEG sensitivity in identifying interictal abnormalities. With scarce recent publications on this common practice, our findings showed the minimal contribution to EEG interpretation outcomes and are of questionable use in its current format. We suggest including one cycle of spontaneous sleep EEG performed at the outpatient department as another alternative and additional option to the existent video telemetry EEG. This should be selectively directed to patients with a history suggestive of epilepsy if their awake EEGs are normal.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Cologan V, Schabus M, Ledoux D, Moonen G, Maquet P, Laureys S. Sleep in disorders of consciousness. Sleep Med Rev 2010 Apr;14(2):97-105.
- 2. Berry R, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. The AASM manual for the scoring of sleep and associated events rules, terminology and technical specification. American Academy of Sleep Medicine; 2015; version 2.2.
- 3. Watanabe T, Kan S, Koike T, Misaki M, Konishi S, Miyauchi S, et al. Network-dependent modulation of brain activity during sleep. Neuroimage 2014 Sep;98:1-10.
- 4. Husain AM, Sinha SR. Nocturnal epilepsy in adults. J Clin Neurophysiol 2011 Apr;28(2):141-145.
- 5. Halász P. How Sleep activates epileptic networks?. Epilepsy Research and Treatment. 2013;2013:19.
- 6. Provini F, Plazzi G, Lugaresi E. From nocturnal paroxysmal dystonia to nocturnal frontal lobe epilepsy. Clinical Neurophysiology 2000;111(Suppl.2): S2-S8.
- 7. Gibbs SA, Proserpio P, Terzaghi M, Pigorini A, Sarasso S, Lo Russo G, et al. Sleep-related epileptic behaviors and non-REM-related parasomnias: Insights from stereo-EEG. Sleep Med Rev 2016 Feb;25:4-20.
- 8. Minecan D, Natarajan A, Marzec M, Malow B. Relationship of epileptic seizures to sleep stage and sleep depth. Sleep 2002 Dec;25(8):899-904.
- 9. Kotagal P. The relationship between sleep and epilepsy. Semin Pediatr Neurol 2001 Dec;8(4):241-250.
- 10. Faulkner HJ, Arima H, Mohamed A. The utility of prolonged outpatient ambulatory EEG. Seizure 2012 Sep;21(7):491-495.
- 11. Baheti NN, Radhakrishnan A, Radhakrishnan K. A critical appraisal on the utility of long-term video-EEG monitoring in older adults. Epilepsy Res 2011 Nov;97(1-2):12-19.
- 12. Alving J, Beniczky S. Diagnostic usefulness and duration of the inpatient long-term video-EEG monitoring: findings in patients extensively investigated before the monitoring. Seizure 2009 Sep;18(7):470-473.
- 13. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure 2008 Jan;17(1):34-41.
- 14. Alsaadi TM, Thieman C, Shatzel A, Farias S. Video-EEG telemetry can be a crucial tool for neurologists experienced in epilepsy when diagnosing seizure disorders. Seizure 2004 Jan;13(1):32-34.
- 15. Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, Eds. Electroencephalography:Basic Principles, Clinical Application and Related Fields. 4th ed. Baltimore: Lippincott Williams & Wilkins; 1999. p. 163.
- 16. Guideline one: minimum technical requirements for performing clinical electroencephalography. American Electroencephalographic Society 2008 [cited 2017 Feb 21]. Available from: https://www.acns.org/pdf/guidelines/Guideline-1.pdf.
- 17. British Society for Clinical Neurophysiology. About Clinical Neurophysiology [cited 2017 Feb 21]. Available from: http://www.bscn.org.uk/.
- 18. Beniczky S, Aurlien H, Brøgger JC, Fuglsang-Frederiksen A, Martins-da-Silva A, Trinka E, et al. Standardized computer-based organized reporting of EEG: SCORE. Epilepsia 2013 Jun;54(6):1112-1124.
- 19. Al-Rawas SF, Poothrikovil RP, Abdelbasit KM, Delamont RS. The Correlation between electroencephalography amplitude and interictal abnormalities: Audit study. Sultan Qaboos Univ Med J 2014 Nov;14(4):e473-e477.
- 20. Miskin C, Carvalho KS, Valencia I, Legido A, Khurana DS. EEG Duration: The Long and the Short of It. J Child Neurol 2015 Nov;30(13):1767-1769.