It is suggested that a minimum of eight hours of sleep per night is needed for normal regulation of metabolism.1 Slow-wave sleep is associated with a decreased use of glucose mediated by the brain, stimulation of growth hormone release, inhibition of cortisol secretion, and a decrease in sympathetic activity.2 Experimental studies in healthy adults have shown that partial sleep restriction (4–5 hour sleep/night over one to 14 nights) rapidly reduces glucose tolerance and insulin sensitivity.3 Furthermore, epidemiological and laboratory-based studies support the role of short sleep duration in the development of obesity and type 2 diabetes mellitus (T2DM).4 Experimental sleep fragmentation studies have demonstrated that in addition to duration, the quality and architecture of sleep may influence metabolism and body weight regulation.5 Selectively suppressing slow wave sleep (SWS) without affecting total sleep time (TST) results in reduced glucose tolerance and insulin sensitivity.6 Sleep reduction has become a common behavior in modern society. The pathophysiology linking sleep deprivation and disturbances with glucose intolerance could be explained by an increase in sympathetic activity, activation of inflammatory pathways and changes in adipokine profiles.7
Diabetes may be a consequence of sleep breathing disorders, or possibly both disorders are caused by a common factor; obesity.8 Cross-sectional studies demonstrated relationships between obstructive sleep apnea syndrome (OSAS) and fasting insulin and insulin resistance, and between OSAS and overt diabetes.9 Snoring, which is a common symptom of OSAS, has also been shown to predict the onset of diabetes in men and women.10,11 A trend toward decreasing sleep duration has paralleled the obesity epidemic. Furthermore, many studies now support an inverse linear relationship between short sleep duration and body mass index (BMI). This would raise the possibility of a casual relationship between habitual sleep duration and metabolic disorders.12
Despite studies linking habitual sleep deprivation and obesity, there is no study comparing the sleep duration of patients with T2DM and sex- and age-matched controls. We hypothesize that sleep duration would be associated with T2DM independent of OSA. Therefore, the aim of the study was to evaluate the sleep habits of T2DM patients and controls.
Methods
This is a case-control substudy of Oman Diabetes Study (n = 1182).13 Patients with T2DM from the Oman Diabetes Study were recruited from the Diabetes and the Family Medicine clinics or as in-patients from Sultan Qaboos University Hospital. Patients were Omani adult men and women aged > 18 years old with T2DM on different treatment regimes (diet, oral hypoglycemic agent, insulin). Participants were excluded from the study if they had any major neurological disorders, schizophrenia or other psychotic illnesses, or cancer treatment within the preceding year. Pregnant women were also excluded.
Age- and sex-matched healthy Omani subjects free of any cardiometabolic diseases were recruited from the local community. They were recruited following visits to the general medical practice, dermatology, and child spacing clinics. An initial screening of a general medical history and physical examinations were performed. Eligible subjects were then asked to fast overnight and report the following morning to measure their glucose levels. Patients were included as controls using the American Diabetes Association (ADA) criteria with a fasting serum glucose level of < 5.6 mmol/L considered as normoglycemia and glycated hemoglobin (HbA1c) of < 5.8%.
Case and control group patients were asked to fill in sleep questionnaires (the Arabic versions of the Berlin Questionnaire14 and Epworth Sleepiness Scale (ESS)15) to determine their sleep habits. They were also given a diary to mark their sleep and wake time. Participants were asked to report the number of hours per night they had slept over the past seven days and were asked separately about sleep duration on weeknights (Saturday to Wednesday) and at the weekend (Thursday and Friday). Both groups were asked to come to the hospital fasting for blood collections to measure their fasting glucose, HbA1c levels, and lipid profile. Demographic (age and sex), and anthropometric (height, weight, and waist and hip circumference) were also collected from all subjects. Participants were informed about the project, and written consents were obtained. The study was approved by the Ethics and Research Committee of the College of Medicine, Sultan Qaboos University.
Data were analyzed using SPSS Statistics (SPSS Statistics Inc., Chicago, US) version 20. The independent t-test was used to calculate the mean difference between both groups. The duration of night sleep was categorized into two groups using a cut-off of six hours. Binary logistic regression was performed to determine the association between nocturnal sleep duration and diabetes using lipids and BMI as confounders.
Results
Table 1: Case and control group biochemical and sleep characteristics.
|
Age, years |
49.5±8.6 |
50.7±8.1 |
0.170 |
|
BMI, kg/m2 |
29.7±6.5 |
32.1±9.9 |
0.006 |
|
Biochemical parameters |
|
Fasting glucose, mmol/L |
5.2±0.6 |
9.4±3.9 |
< 0.001 |
|
HbA1c, % |
5.8±0.5 |
8.8±1.8 |
< 0.001 |
|
TCHOL, mmol/L |
5.4±0.9 |
4.9±1.0 |
< 0.001 |
|
HDL, mmol/L |
1.2±0.3 |
1.2±0.4 |
0.191 |
|
TG, mmol/L |
1.4±0.8 |
1.8±0.9 |
< 0.001 |
|
LDL, mmol/L |
3. 5±0.9 |
3.0±0.9 |
< 0.001 |
|
Sleep duration and ESS |
|
Noturnal sleep, hours |
6.4±1.3 |
6.1±1.5 |
0.033 |
|
Naps, hours |
1.1±0.7 |
1.1±0.9 |
0.581 |
BMI: body mass index; TCHOL: total cholesterol; HDL: high-density lipoprotein; TG: triglyceride; LDL: low-density lipoprotein; ESS: Epworth Sleepiness Scale; HbA1c: glycated hemoglobin.
Only 580 (n = 234 T2DM and n = 346 control patients) of 1182 answered the sleep questionnaires. Our study used the data from 188 patients with T2DM (age: 50.7±8.1 years; BMI: 32.1±9.9 kg/m2; males: n = 95, 50.5%) and 187 healthy controls (age: 49.5±8.6 years; BMI: 27.7±6.5 kg/m2; males: n = 78, 41.7%). The rest were excluded due to incomplete data or not matching with age.
The study selected age- and sex-matched healthy Omani subjects. The gender distribution in both groups was similar (T2DM: males n = 95, 50.5% and females n = 93, 49.5%; controls: males n = 78, 41.7% and females n = 109, 58.2%). One male subject from the control group refused to take part in the study.
The descriptive statistics of the study sample is shown in Table 1. Patients with diabetes had significantly higher BMI, fasting glucose, and triglycerides (TG) and low total cholesterol and low density lipoprotein (LDL) compared to controls. high density lipoprotein (HDL) levels were similar in both groups. There was no association between diabetes and gender (χ2 = 3.9, p = 0.14).
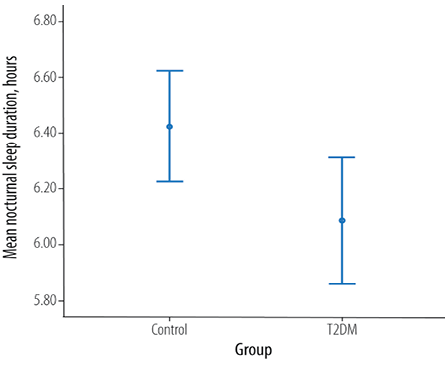
Figure 1: Total nocturnal sleep in patients with type 2 diabetes mellitus (T2DM) and aged-matched healthy controls (p = 0.033).
There was a significant difference between patients with T2DM and the control group in nocturnal sleep duration (p = 0.033) with a mean difference of 0.4 hours. The mean nocturnal sleep duration among the control and T2DM group was 6.1±1.5 hours and 6.4±1.3 hours, respectively [Figure 1]. There was a significant association between a nocturnal sleep duration of six hours and diabetes (χ2 = 14.0; p = 0.0001).
Table 2: Binary logistic regression of the association between sleep duration and lipid profile in patients with T2DM.
|
HbA1c, % |
0.004 |
0.001 |
|
TCHOL, mmol/L |
2.7 |
0.031 |
|
HDL, mmol/L |
1.8 |
0.262 |
|
TG, mmol/L |
0.5 |
0.221 |
|
LDL, mmol/L |
2.6 |
0.039 |
HbA1c: glycated hemoglobin; TCHOL: total cholesterol; HDL: high-density lipoprotein; TG: triglyceride; LDL: low-density lipoprotein.
Binary logistic regression for analysis of risk factors for diabetes showed maximum risk with
< 6 hours sleep per night followed by high cholesterol and LDL [Table 2]. There was 3.9 times more risk of developing diabetes if patients slept for < 6 hours at night (p = 0.039). There was 2.7 times more risk of developing diabetes if total cholesterol was high
(p = 0.031) and 2.6 times (p = 0.039) if LDL was high. Low HDL and high TG did not carry significant risk for developing diabetes. When the present binary logistic model was tested against BMI, it showed that a high BMI did not affect the risk of diabetes (β = 1.9; p = 0.262) and association of night sleep duration and lipid parameters with risk of diabetes was independent of high BMI.
There was no significant correlation between hip/waist ratio and sleep duration (p = 0.211) or daytime nap (p = 0.311) in the T2DM group or in the control group (p = 0.812 and p = 0.231, respectively).
There was no significant difference in daytime sleepiness (measured by the ESS) and daytime naps between the T2DM and control groups (p = 0.452 and p = 0.581, respectively). Patients with T2DM who slept less than six hours per night had significantly prolonged daytime naps (1.2±0.8 hours) compared to those who slept for six or more hours a night (0.8±0.7 hours; p = 0.041).
Table 3: Obstructive sleep apnea (OSA) risk category in patients with T2DM and control group (determined by the Berlin Questionnaire).
|
Male |
|
|
|
|
Low |
60 |
64 |
124 |
|
High |
18 |
31 |
49 |
|
Total |
78 |
95 |
173 |
|
Female |
|
|
|
|
Low |
77 |
59 |
136 |
|
High |
32 |
34 |
66 |
|
Total |
109 |
93 |
202 |
|
Combined |
|
|
|
|
Low |
137 |
123 |
260 |
|
High |
50 |
65 |
115 |
The number of diabetic men at high risk of OSA was 31 compared to 18 control men. Similarly, there were 34 women with diabetes at high risk of OSA compared to 32 control women [Table 3]. There was no significant difference between patients with T2DM and the control group in the assessment of the risk of obstructive sleep apnea (p = 0.061). There was no association between increased OSA risk and diabetes in males (χ2 = 1.9; p = 0.110) and females (χ2 = 1.2;
p = 0.171).
The study demonstrated an association between habitual short nocturnal sleep with T2DM when compared to healthy controls. Furthermore, the association with T2DM was much stronger among diabetic patients who slept less than six hours per night. Although some studies have reported that experimentally restricted and fragmented sleep may lead to decreased insulin sensitivity, these studies had a small sample size.1,3 Other prospective studies have also reported an association between sleep deprivation and metabolic syndrome in adults.16
Specifically, we observed an association between habitual sleep restriction and T2DM in a case-control design. Ayas et al,17 found that although short sleep was predictive of diabetes over 10 years in initially healthy women (n = 70,026), this effect was no longer significant after adjustment for BMI at baseline. The current sample consisted of a mixed population of age- and sex-matched men and women. Nevertheless, Yaggi et al,18 found in a longitudinal study using self-reported sleep habits, that both a long and short sleep duration was associated with T2DM independent of confounding factors.
Other studies have reported an association between short sleep duration and decreased insulin sensitivity in healthy men. One study found a shorter sleep duration may impair insulin sensitivity and beta-cell function in nondiabetic white men.19 Similarly, they used self-reported sleep habits to calculate nocturnal sleep duration and daytime napping. Another study by Sung et al,20 reported that sleep duration was not associated with metabolic outcomes, and showed limited associations with lipid profiles. They studied adolescents aged between 10–17 years. In our study, the mean age was 50.7 years for patients with T2DM and 49.5 years for patients in the control group. This suggests age might be a confounding factor. However, our study was a case-control cohort that took into account the effect of age and might have eliminated this confounding factor to some extent.
Sleep loss can contribute to T2DM by interfering with nighttime glucose regulation directly, mainly due to a shorter duration of slow wave sleep or by disturbance of appetite with an increased risk for obesity.21 Orexin-containing neurons are thought to maintain arousals and increase food intake.22 Studies have shown that sleep restriction increases self-reported hunger and calorie consumption and
could reduce daytime physical activity caused
by fatigue.23
In our study, patients with T2DM who slept less than six hours per night had prolonged daytime naps but were not significantly sleepy using the ESS compared to controls. Given the confounding relationship between truncal obesity and glucose intolerance, there was no significant correlation between nocturnal or daytime sleep duration and hip/waist ratio with BMI. Therefore, obesity as a confounding risk factor for T2DM was removed. However, using binary logistic regression, nocturnal sleep duration was significantly correlated with T2DM in the presence of high levels of total and low-density lipoprotein cholesterol. Nevertheless, the control group had a higher LDL cholesterol, and that might be because most patients with T2DM in Oman take lipid-lowering drugs.24,25
OSA is a known independent risk factor for T2DM and patients with OSA are known to have coexisting risk factors for metabolic syndrome such as obesity.8,9,26 In our study, there was no difference between the control and T2DM groups in the risk of having obstructive sleep apnea using the Berlin Questionnaire. Our study may contradict previous studies that revealed sex differences in the prevalence of OSA and the association of T2DM and OSA. Cross-sectional studies have shown that 30% of patients with OSA have T2DM and 20% of these are diagnosed with glucose intolerance.27 While this study used nocturnal polysomnography to reach the diagnosis of OSA, we used the Berlin Questionnaire. Nevertheless, our findings would strengthen the association between T2DM and habitual chronic sleep deprivation independent of OSA.
It is important to note that our study quantified sleep duration based on self-reports. Relative to sleep times based on actigraphy measures, self-reported sleep duration tends to be overestimated and have poor internal validity. Daytime/afternoon naps are common practice among our local population, and it may have an effect on night sleep duration. In addition, we used the Berlin Questionnaire to stratify the risk of OSA rather than polysomnography and that may not be a sensitive method to ascertain the diagnosis of sleep breathing disorders in the study groups, though this questionnaire has been used widely in sleep research as a screening tool. The genetic element, which could be a strong confounder, of T2DM in the sample population was addressed and confirms the familial aggregation of diabetes among the Omani population.28 Finally, physical activity and calorie intake were not calculated in the current sample and maybe confounding factors with a great impact on the development of T2DM.
The principal findings of the current study suggest that chronic sleep deprivation may predispose individuals to T2DM. Future, community-wide studies exploring the link between habitual sleep patterns, particularly poor sleep hygiene, and the cardiometabolic disorders, are needed using objective methods such as actigraphy and polysomnography to study sleep duration and sleep-related disorders.
The authors declared no conflicts of interest. The study was funded by The Research Council (TRC) of Oman.