|
Abstract
Objective: To assess the relationship of bacterial and fungal contamination on used surgical masks worn by the hospital personnel and microbial air quality in their working wards.
Methods: This is a cross-sectional study of 230 used surgical masks collected from 214 hospital personnel, and 215 indoor air samples collected from their working wards to culture for bacterial and fungal counts. This study was carried out at the hospital in Bangkok. Group or genus of isolated bacteria and fungi were preliminarily identified by Gram’s stain and lacto-phenol cotton blue. Data were analyzed using paired t-test and Pearson’s correlation coefficient at the significant level of p<0.050.
Results: Means and standard deviation of bacterial and fungal contamination on inside area of the used masks were 47 ± 56 and 15 ± 9 cfu/ml/piece, and on outside area were 166 ± 199 and 34 ± 18 cfu/ml/piece, respectively, p<0.001. The bacterial and fungal contamination on used masks from hospital personnel working in the male and female medical wards and out-patient department, as well as the bacterial and fungal counts of the indoor air sample collected from the same area were relatively higher than the other wards. The predominant isolated bacteria and fungi contaminated on inside and outside areas of the used masks and air samples were similar (Staphylococcus spp. and Aspergillus spp.; respectively). For its relationship, results found that bacterial and fungal counts in air samples showed significantly positive correlation with the bacterial contamination load on outside area of the used masks, r=0.16, p=0.018 and r=0.21, p=0.003, respectively.
Conclusion: High bacterial contamination on outside area of the used masks was demonstrated, and it showed a significant correlation with microbial air quality of working wards.
Keywords: Microbial contamination; Hospital personnel; Microbial air quality.
Introduction
In hospitals, the main risk of infection for healthcare workers (HCWs) are the transmission from patients, contaminated instruments or equipments and the hospital environments.1-6 Patients and HCWs who have no symptoms of the disease or carriers of chronic disease are both potential sources and hosts of infectious agents. The sources of infectious agent are the normal endogenous microbial flora of the patients, HCWs, and the environmental sources such as air, water and the devices that have become contaminated.2-4 Indoor air quality in healthcare settings is an important issue. It affects the healthcare personnel based on evidence data about exposure to indoor air pollution, particularly in relation to allergies, asthma and respiratory diseases.2-4,7,8 The aerosol particles of biological origin e.g., viruses, bacteria and fungal spores, have been associated with respiratory allergies, asthma, and several air-borne infections including influenza, tuberculosis, measles, mumps, chicken pox, and aspergillosis.2,7-9 The adverse health effects of the biologic agents depend not only on the mass or number of the inhaled particles, but also on the infectivity of agents.10,11
The disposable masks including surgical masks and N95 were originally developed to filter droplets containing microorganisms expelled from the mouth and nose. It was introduced by World Health Organization (WHO), and National Institute for Occupational Safety and Health (NIOSH) to protect the patients or HCWs from the risk of various respiratory infections.12-14 But some factors should be considered, especially the selection of a mask, which has different pore sizes to filter particles for preventing the spread of air-borne pathogens and the mask used behaviors.15-18 Hospital personnel, especially nurses are at risk to acquire the infection during nursing care and coming in contact with patients. Evidences showed that the surgical mask might not be enough to protect the person from air-borne pathogens and might also be the source of air-borne or droplet infection.15,19,20 This study aimed to investigate the microbial contamination on used disposable surgical masks among hospital personnel and microbial air quality in their working wards and its relationship. These are useful in developing prevention and control programs among HCWs.
Methods
A cross-sectional study was conducted to assess the bacterial and fungal contamination on 230 used surgical masks (Face mask ear-loop 3 Ply US FDA 510 K) from 214 hospital personnel. The inside and outside area of the masks were analysed. The partakers were males and females aged ≥18 years, and have voluntarily participated and signed the consent form. Furthermore, 215 indoor air samples were collected from their working wards in the same days during the 2-month observation to investigate the microbial air quality (bacterial and fungal counts). The study was conducted with the ethical approval of the ethical committee of Mahidol University (Reference No. MU-IRB 2009/ 130.2306).
The 214 hospital personnel were working from different departments such as: male and female medical wards, intensive care unit, emergency room, operating room and medical outpatient department of the governmental hospital in Bangkok. Their used surgical masks, which were 230 in total, were collected to culture the bacterial and fungal counts. Inside and outside areas of the masks were separated by sterile technique and put in a sterile container consisting of trypticase soy broth for 20 minutes. A spread plate method was used for determining total bacterial and fungal counts. General bacteria are cultivated in a plate count agar (PCA) and general fungi are cultivated in a Sabouraud 4% dextrose agar (SDA). The plates were incubated at 37ºC for 48 hours to get the bacterial counts, and incubated at room temperature for 5 days for fungal counts. Observation was done daily. After counting the isolated bacteria, fungi were preliminarily identified by Gram’s stain and microscopic morphology (lacto-phenol cotton blue) following Larone (1995).21
A total of 215 indoor air samples were collected from the participants working wards including: 40 samples from male medical ward, 35 samples from female medical ward, 38 samples from emergency room (ER), 47 samples from operation room (OR), 31 samples from intensive care unit (ICU), and 24 samples from out-patient department (OPD). Sampling was done during 12:00 to 15:00 p.m. every Monday, Wednesday and Friday for two months using the Millipore Air Tester to assess bacterial and fungal counts. Air sampling collection points includes nurse station, central area of patient room and patient bedside, and the examination room. The nurse station has air conditioner, while the patient section is naturally ventilated and supplemented with electric ceiling fans for better air circulation. The temperature in the nurse station was about 25ºC, and the patient room was about 31º to 34ºC.
The Millipore Air Tester system is based on the Anderson principle that uses a sieve with about 1,000 microperforation, which reduces the potential for overlapping colonies and minimizes the desiccation of the medium. The tester is small enough to be used in confined spaces, but powerful enough to sample up to 1000 liters in just seven minutes. In this study, 250 liters of air were collected. General bacteria were cultured in PCA plates at 37ºC for 48 hrs, and general fungi were cultured in SDA plates at room temperature for 5 days with daily observation. After incubation, the bacterial and fungal colonies were counted and calculated to express as colony forming unit/m3 (cfu/m3) by a formula:
Total counts (colony forming unit/m3 or cfu/m3) = [Total colonies × 1000]/250
The isolated bacteria and fungi were preliminarily identified by Gram’s stain and microscopic morphology (lacto-phenol cotton blue) following Larone (1995).21
Data were analyzed using descriptive statistics including percentage, mean and standard deviation (SD) for describing bacterial and fungal counts. Paired t-test was used to compare between mean of microbial contamination on outside and inside areas of used masks. The correlation between bacterial and fungal counts in air samples collected from working wards and the loads of bacterial and fungal contamination on the used masks was analyzed using Pearson’s correlation coefficient. The significant level was set at p<0.050.
Results
A total of 230 used masks were collected from 214 personnel to assess the bacterial and fungal contamination. Results revealed that the mean ± SD of bacterial contamination on inside area of the used masks was 47 ± 56 cfu/ml/piece and 166 ± 199 cfu/ml/piece from outside area. It was significantly different, p<0.001. The used masks from hospital personnel working in male and female medical wards had relatively higher bacterial contamination than the other wards (Table 1). Most isolated bacteria contaminated on inside and outside areas of the used masks were Staphylococcus spp. (34% and 41%, respectively) and Pseudomonas spp. (34% and 38%, respectively). Details are presented in (Table 1). For fungal contamination, mean ± SD on outside area of the used masks was significantly higher than the inside area (34 ± 18 and 15 ± 9 cfu/ml/piece), p<0.001. The used masks from hospital personnel working in OPD and the female medical ward had relatively higher fungal contamination than the other wards (Table 2). Fungi found on inside and outside areas of the used masks were Aspergillus spp. (37% and 44%, respectively), and Penicillum spp. (31% and 25%, respectively). (Table 2)
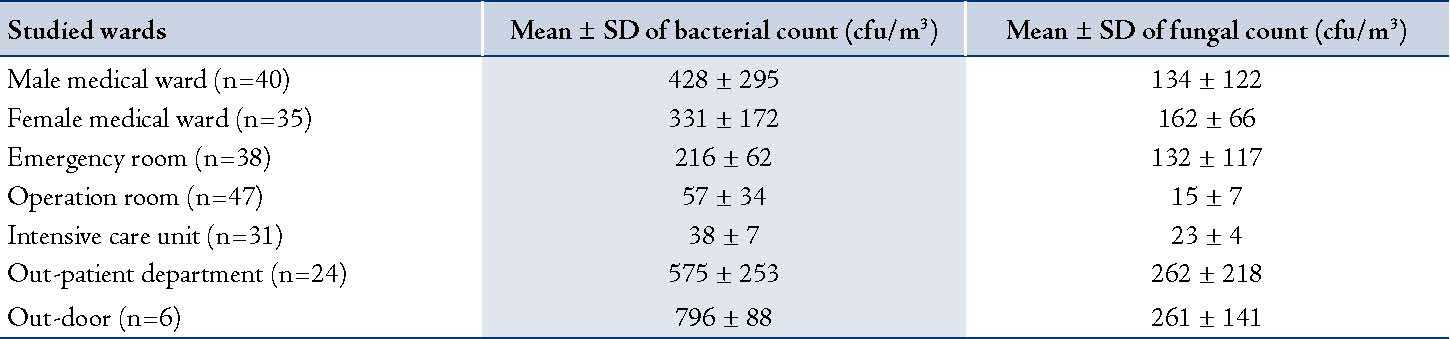
Analysis of 215 indoor air samples collected from the studied wards revealed that the OPD had the highest bacterial and fungal mean counts (576 ± 254 cfu/m3 and 263 ± 219 cfu/m3; respectively). While the intensive care unit had the lowest bacterial mean count (38 ± 8 cfu/m3) and the operation room had the lowest fungal mean count (19 ± 7 cfu/m3). Air samples collected from OPD, male and female medical wards had relatively higher average bacterial and fungal counts than those collected from the other wards. Moreover, isolated bacteria and fungi were preliminarily identified; it was found that the predominant bacteria and fungi were Staphylococcus spp. (57%) and Penicillium spp. (39%) and Aspergillus spp. (31%), respectively. Details are shown in table 3.
Table 1: Bacterial contamination on used surgical masks (cfu/ml/piece) by studied wards: inside and outside areas of the used masks (n = 230).
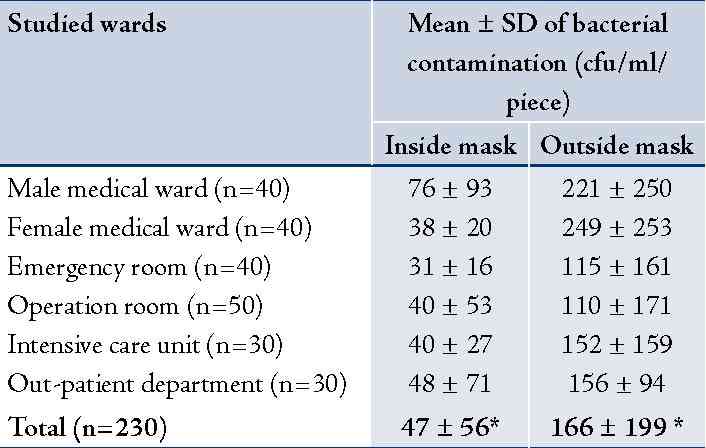
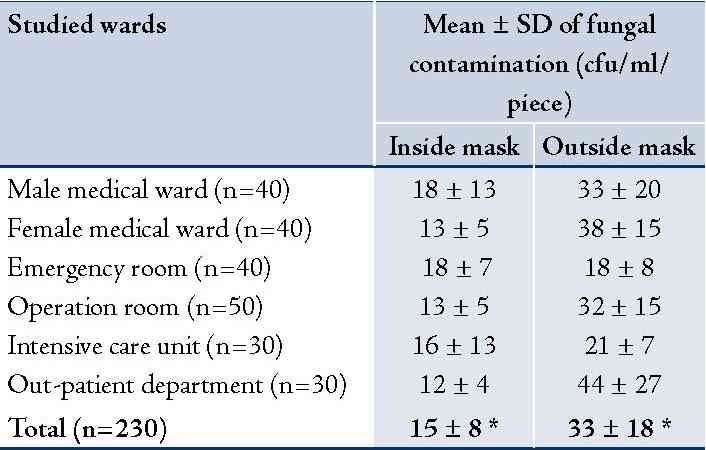
Table 3: Means and standard deviation of bacterial and fungal counts of air samples collected from studied wards.
The correlation between bacterial and fungal counts in air samples collected from working wards and the microbial contamination loads on the used masks (inside or outside area) was analyzed. It was found that bacterial and fungal counts in air samples showed significantly positive correlation with the bacterial contamination found on outside area only of the used masks (r=0.16, p=0.018 and r = 0.21, p=0.003; respectively). The details are shown in table 4.
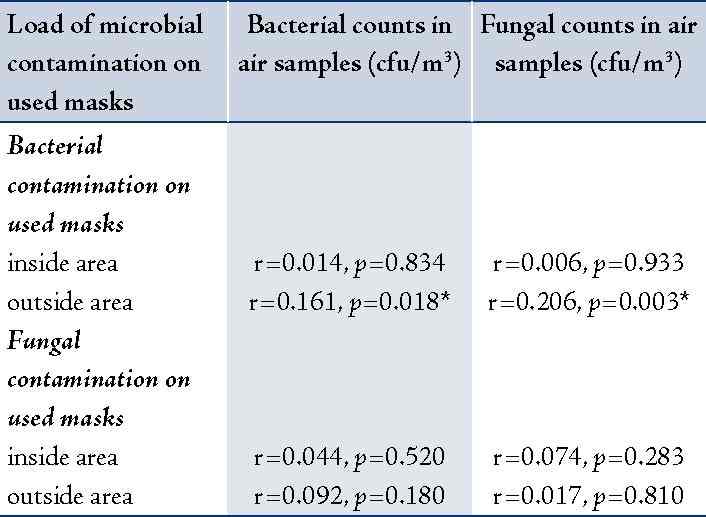
* Statistically significant correlation at α = 0.05
Discussion
This study was a short-term assessment of bacterial and fungal contamination on the used surgical masks among hospital personnel and microbial air quality in their working wards. The disposable masks were originally developed to filter droplets containing microorganisms expelled from the mouth and nose, and probably to protect the human respiratory system from fine air-borne particles that are known to be associated with various respiratory diseases.12-14 WHO and NIOSH recommended the optimal types of disposable masks for different purposes. Generally, the surgical mask is designed to prevent microorganisms from the nose and mouth of the wearer from spreading to others. It is not efficient to filter particles of some infectious agents, especially M. tuberculosis and some viral particles.15 The present study found that both bacterial and fungal contamination on outside areas of the used masks were significantly higher than the inside areas (166 ± 199 vs. 47 ± 56 cfu/ml/piece, p<0.001 and 34 ± 18 vs. 15 ± 9 cfu/ml/piece, p<0.001; respectively). This evidence supported that the studied surgical masks could filter most of contaminated bacteria and fungi in air of the working wards. However, our study included only one type of surgical mask and did not detect the viral contamination. Also, some used mask behaviors probably increased the microbial contamination on the masks.22 When isolated colonies were randomly selected to identify bacterial groups/genus and fungal groups/genus by Gram’s stain and lacto-phenol cotton blue staining, respectively; it was found that the predominant isolates contaminated on the outside of the used masks were Staphylococcus aureus (41%) and Pseudomonas spp. (38%), and the most isolated fungi were Aspergillus spp. (44%), and Penicillum spp. (25%).
The microbial indoor air quality assessment in this present study revealed that OPD had the highest average of bacterial and fungal counts (576 ± 254 cfu/m3 and 263 ± 219 cfu/m3), while ICU and OR had the lowest average of bacterial count and fungal count, respectively. Air samples collected from OPD, male and female medical wards had relatively higher average of bacterial and fungal counts than the other wards. This evidence was similar to the bacterial and fungal contamination on used masks. When isolated colonies were preliminarily identified, it was found that the predominant bacteria were Staphylococcus spp. (57%) and the predominant fungi were Penicillium spp. (39%) and Aspergillus spp. (31%). Similar bacterial and fungal groups were found in used masks and indoor air of the working wards. This evidence probably supported the accumulation of air-borne bacteria and fungi on the used masks.
When correlation was analyzed between microbial air quality (bacterial and fungal counts) in working wards and the microbial contamination loads on the used masks (inside and outside area of the used masks), it was found that only bacterial and fungal counts in air showed significantly positive correlation with the bacterial contamination load on outside area of the used masks (r=0.16, p=0.018 and r=0.21, p=0.003; respectively). If we could reduce bacterial and fungal counts in indoor air in the working wards, the bacterial contamination load on the outside area of the masks will probably decreased. Our previous study demonstrated that the cleaning of local exhaust fans, fans and air conditioners, and managing everything that blocked the natural air from the windows and doors could improve the ward ventilation and reduce the microbial counts in indoor air.3 Bio aerosol is an important biological component in the hospital environments. Infectious agents can be carried as airborne particles or droplet nuclei then spread quickly to people. Most infections are due to inhalation of droplet nuclei from 1 to 5 micron range.15,20 The bacteria can settle in the lungs and grow causing an infection. The American Conference of Governmental Industrial Hygienists (ACGIH) recommended that the optimal level of bacterial counts or fungal counts in indoor air should be less than 500 cfu/m3. The high bacterial count or fungal count in indoor air of general workplaces was an indication of poor ventilation and congestion.23 However, World Health Organization,24 suggested that the microbial counts in the general offices should be less than 300 cfu/m3 and for individuals or patients with immune-suppression, it should be less than 100 cfu/m3. The microbial counts in indoor air depends on several variables, such as velocity, humidity, temperature, ventilation, number of occupants, people’s activities, a concentration of particulate matter or dust, and the outdoor air quality.23,25,26 To prevent the microbial growth, avoidance of wet surfaces, keeping relative humidity levels below 70%, effective filtration of particulates, proper HVAC system operation and maintenance, and good housekeeping should be implemented.27,28
Conclusion
This present study revealed that the high bacterial contamination on the outside area of the used surgical masks had significantly positive correlation with bacterial and fungal counts found in air samples that were collected from the working wards. To reduce the load of bacterial contamination on the used masks, the hospital environments, especially microbial air quality in the working wards should be improved.
Acknowledgements
This study was supported for publication by the China Medical Board, Faculty of Public Health, Mahidol University, Bangkok, Thailand. Finally, we would like to acknowledge the Director of the studied hospital and also wish to extend our deep appreciation to all participants in the present study.
References
1. Amoran OE. Occupational exposure, risk perception and access to prophylaxis for HIV/AIDS infection among healthcare workers in Northern Nigeria. Br J Med Med Res 2013 Feb;3(2):275-287 .
2. Delclos GL, Gimeno D, Arif AA, Burau KD, Carson A, Lusk C, et al. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med 2007 Apr;175(7):667-675.
3. Luksamijarulkul P, Khumsri J, Vatthanasomboon P, Aiumlaor P. Improving tuberculosis infection control practice and microbial air quality in a general hospital after intervention. Asian Pac J Trop Med 2009 Apr;2(2):39-46.
4. Sepkowitz KA, Eisenberg L. Occupational deaths among healthcare workers. Emerg Infect Dis 2005 Jul;11(7):1003-1008.
5. Al Jahdhami I. Latent tuberculosis in healthcare workers: time to act. Oman Med J 2013 Mar;28(2):146-148.
6. Luksamijarulkul P, Kiennukul N, Vatthanasomboon P. Laboratory facility design and microbial indoor air quality in selected hospital laboratories. Southeast Asian J Trop Med Public Health 2014 May;45(3):746-755.
7. Bennett A, Parks S. Microbial aerosol generation during laboratory accidents and subsequent risk assessment. J Appl Microbiol 2006 Apr;100(4):658-663.
8. Luksamijarulkul P, Panya N, Sujirarat D, Thaweboon S. Microbial air quality and standard precaution practice in a hospital dental clinic. J Med Assoc Thai 2009 Dec;92(Suppl 7):S148-S155.
9. Bonetta S, Bonetta S, Mosso S, Sampò S, Carraro E. Assessment of microbiological indoor air quality in an Italian office building equipped with an HVAC system. Environ Monit Assess 2010 Feb;161(1-4):473-483.
10. Tunevall TG, Jörbeck H. Influence of wearing masks on the density of airborne bacteria in the vicinity of the surgical wound. Eur J Surg 1992 May;158(5):263-266.
11. Letts RM, Doermer E. Conversation in the operating theater as a cause of air-borne bacterial contamination. Am J Bone Joint Surg 1983;65:357-362.
12. Huang C, Willeke K, Qian Y, Grinshpun S, Ulevicius V. Method for measuring the spatial variability of aerosol penetration through respirator filters. Am Ind Hyg Assoc J 1998 Jul;59(7):461-465.
13. National Institute for Occupational Safety and Health (NIOSH).42CFR 84 Respiratory Protective devices: Final rules and notice. Federal Register 60:110. U.S. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 1997.
14. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005 Dec;54(RR-17):1-141.
15. Willeke K, Qian Y, Donnelly J, Grinshpun S, Ulevicius V. Penetration of airborne microorganisms through a surgical mask and a dust/mist respirator. Am Ind Hyg Assoc J 1996 Apr;57(4):348-355.
16. McLure HA, Talboys CA, Yentis SM, Azadian BS. Surgical face masks and downward dispersal of bacteria. Anaesthesia 1998 Jul;53(7):624-626.
17. Alwitry A, Jackson E, Chen H, Holden R. The use of surgical facemasks during cataract surgery: is it necessary? Br J Ophthalmol 2002 Sep;86(9):975-977.
18. Luksamijarulkul P, Supapvanit C, Loosereewanich P, Aiumlaor P. Risk assessment towards tuberculosis among hospital personnel: administrative control, risk exposure, use of protective barriers and microbial air quality. Southeast Asian J Trop Med Public Health 2004 Dec;35(4):1005-1011.
19. Harber P, Barnhart S, Boehlecke BA, Beckett WS, Gerrity T, McDiarmid MA, et al; American Thoracic Society. Respiratory protection guidelines. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, March 1996. Am J Respir Crit Care Med 1996 Oct;154(4 Pt 1):1153-1165.
20. Fennelly KP. Personal respiratory protection against Mycobacterium tuberculosis. Clin Chest Med 1997 Mar;18(1):1-17.
21. Larone DH. Medically important fungi: a guide to identification. 3rd ed. Washington DC: American Society of Microbiology Press; 1995.
22. Kretzer EK, Larson EL. Behavioral interventions to improve infection control practices. Am J Infect Control 1998 Jun;26(3):245-253.
23. Seitz TA. NIOSH indoor air quality investigations1971-1988. In: Weekes DM, Gammage RB, editors. Proceedings of the Indoor Air Quality, International Symposium: May 23, 1989; Cincinnati, OH: National Institute for Occupational Safety and Health; 1989 P, 163-171.
24. World Health Organization. Indoor air quality: biological contaminants. Report on a WHO meeting. WHO Reg Publ Eur Ser 1990;31:1-67.
25. Kodama AM, McGee RI. Airborne microbial contaminants in indoor environments. Naturally ventilated and air-conditioned homes. Arch Environ Health 1986 Sep-Oct;41(5):306-311.
26. Jacobs RR. Risk environments. In: Rylander R,Jacobs RR, editors. Organic dusts: exposure, effects and prevention. Boca Raton, Fl: Lewis Publisher; 1994 P, 3-15.
27. Li Y, Leung GM, Tang JW, Yang X, Chao CY, Lin JZ, et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air 2007 Feb;17(1):2-18.
28. Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface 2009 Dec;6(Suppl 6):S737-S746.
|