Diabetes mellitus (DM) is one of the major chronic diseases of our time, affecting nearly 8.5% of the world’s population as of 2014.1 The percentage of people affected continues to rise with an estimate of 592 million people by 2035.2 The Middle East and North Africa region showed a higher DM prevalence of 9.6% (aged 20–79 years) and the prevalence by 2045 for this region was expected to increase to 12.1%.3 The trend of increasing prevalence of diabetes does not exclude Oman as the prevalence of DM in Oman had risen from 11.6% in 2000 (aged 20+) to 12.3% in 2008 (aged 20+) to 14.5% in 2017.4 The 2016 annual statistics book contained 89 246 patients with diabetes of whom 6442 new cases were diagnosed in 2016.5
The increase in life expectancy of the DM population makes them more prone to develop complications.2 DM is a major factor contributing to cardiomyopathy, nephropathy, peripheral neuropathy, and diabetic retinopathy (DR). DR is defined as the presence of one or more retinal microaneurysms or retinal blot hemorrhages with or without more severe lesions (hard exudates, soft exudates, intra-retinal microvascular abnormalities, venous beading, retinal new vessels, pre-retinal and vitreous hemorrhage, and fibroproliferans).6 DR is a serious condition that can ultimately lead to visual impairment if not diagnosed and managed in time. It affects approximately one-third of DM patients.3 DR is considered the number one cause of blindness among working-age adults.7
The prevalence of DR worldwide was 34.6%, according to a meta-analysis that reviewed multiple studies between 1980 and 2008.8 The estimated prevalence of DR in Oman was between 14.5% and 42.2% within DM patients.2 The overall prevalence of DR within type 2 DM (T2DM) patients in Asia was 28%.9 Other neighboring countries have shown a variable pattern. The prevalence of DR in Saudi Arabia was 36.8% for diabetic patients over 50 years of age.10 Moreover, the prevalence of DR in Jordan was 34.1%,11 while in the UAE it was 19%.12 Kuwait seemed to have the highest prevalence of DR with 40%.13 Based on a screening study in Bahrain, 20% of individuals were diagnosed with DR.14 The prevalence in Qatar was reported as 23.5%.15
There are essentially two types of DR, non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). In NPDR there is no formation of new blood vessels; its manifestation can be seen as formation of microaneurysms in the blood vessels as well as macular edema. PDR, which is a more severe form, involves the formation of new blood vessels in the eye that usually leak fluid into the vitreous humor. This leads to scar tissue formation and, eventually, the retina’s detachment from the back of the eye. Finally, the newly formed vessels may interrupt the eye’s normal flow system, which can lead to pressure build-up and can manifest as glaucoma.
The previous meta-analysis also concluded that the world prevalence of PDR was 6.96%.8 In a systemic review of 62 studies of DR in Africa, reviewed between January 1990 and February 2011, the prevalence of PDR among diabetic patients was 0.9–1.3%.16 In a meta-analysis conducted in Asia that included 42 studies from Indian, South Korean, Malaysian, Singaporean, Asian, and Chinese populations, the overall calculated PDR prevalence among the DM population was 6% and 17% among DR patients,9 which is similar to that of Africa. The previous study also showed that the prevalence of NPDR in Asia among T2DM patients was 27%, and among DR patients, the prevalence was 83%. Moreover, a hospital-based study conducted in Oman in 2003 showed a lower DR prevalence of 14.4%; it also showed a NPDR prevalence of 8.6% and a PDR prevalence of 2.66%.17
There are very few studies that investigated the DR prevalence in Oman. In addition, these studies were conducted at a single health center or secondary hospital with a small sample size. Thus, we cannot compare where Oman stands in relation to other countries in terms of DR prevalence.
This is the first study of its kind that targets and investigates the prevalence of DR at a national level over almost 20 years. Determining the prevalence of DR and its subtypes (PDR and NPDR) is important to have a clearer understanding of the burden of this problem.
We sought to determine the prevalence of DR and its subtypes, PDR and NPDR, in the diabetic Omani population.
Methods
We conducted a retrospective cross-sectional study which included 2000 cases obtained through the Ministry of Health (MOH) AlShifaa system. All cases from 2000 to 2017 that met the inclusion criteria were included in the study.
All Omani patients, males and females, aged 18 years or above, and diagnosed with type 2 diabetes were included in the study. Only DR cases that were diagnosed or confirmed by an ophthalmologist were included.
Cases of other ethnic groups were excluded from the study. Moreover, gestational diabetes in pregnant women was not taken into account, and retinopathy of any etiology other than those occurring in patients with T2DM was also excluded.
We used Epi info to calculate the study sample size of 1168 (taking a 99.9% confidence level and 2% acceptable margin of error). The proportion taken was 14.4%, which was the previous low-end estimate of DR in Oman. The population size entered was 89 246, as it is the total number of registered DM patients within the Alshifa database. We added an extra 832 cases. Therefore, a total of 2000 patients were reviewed from December 2018 to March 2019 extracted from 79 medical centers from all Oman’s governorates. Out of those, only 616 met the inclusion criteria and were included in the study.
To minimize bias and ensure generalizability, the patients included in the study were chosen through random sampling. Further stratification was done where percentages of DM for each governorate were taken from the MOH annual statistical book, and a sample size for each governorate was calculated [Figure 1].
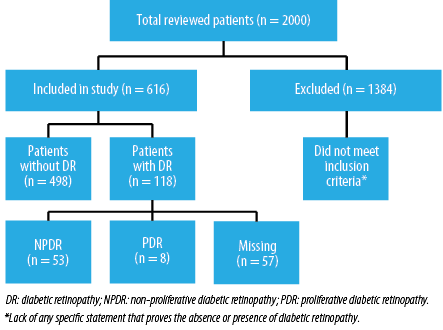
Figure 1: Patients distribution from all governorates in Oman.
Patient ID, sex, date of birth, year of DM diagnosis, and governorate name were extracted from the database. Patient age of DM was calculated from the date of birth and start year of DM. Each record was then reviewed and marked either as normal or retinopathy. For each retinopathy entry, the retinopathy type and the year of diagnosis of retinopathy were entered. The age of patient at the diagnosis of retinopathy was then calculated.
Two-thousand DM cases were analyzed from 1 January 2000 to 31 December 2017 using the ICD 10 code E11. Since not all cases of DR were coded using the appropriate ICD 10 code (H 36.0) in the AlShifa system, each record of T2DM was reviewed for the diagnosis of DR. In other words, DM cases that were not coded for DR within the sample were reviewed to see if these patients were seen by an ophthalmologist and diagnosed with DR by accessing doctors’ notes. The overall prevalence of DR was then calculated with a reasonable level of precision.
Each type of DR was identified by reviewing the case files of diagnosed patients. The prevalence was calculated within both DR and DM samples.
We used SPSS Statistics for statistical analysis (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Further analysis using Chi-square and Fisher’s tests were used to show the association of DR, PDR, and NPDR between the two sexes and age groups. Moreover, cross-tabulation and Fisher’s test were done to see whether there is an association between the age of diagnosis of DM and being diagnosed with DR. Another Fisher’s test was conducted to see the significance between the duration of DM and acquiring DR. The DM duration was defined as the duration between the year of diagnosis of DM and the present year of screening the records (2018). Data was safely stored and encrypted using TrueCrypt software to ensure confidentiality and integrity of patient records.
Ethical approval for the study was obtained from the MOH Research and Ethics Review and Approval Committee on September 2018 (Ethical approval number: MoH/CSR/18/9187).
Table 1: Demographic characteristics of patients.
|
Sex |
|
|
|
|
Males |
250 |
41.0 |
- |
|
Females |
360 |
59.0 |
- |
|
Age, years |
|
|
|
|
≤ 40 |
144 |
23.7 |
- |
|
41–50 |
182 |
29.9 |
- |
|
51–60 |
172 |
28.3 |
- |
|
61–70 |
86 |
14.1 |
- |
|
> 70 |
24 |
3.9 |
- |
|
Governorate |
|
|
|
|
Muscat |
189 |
30.9 |
388 |
|
Al Batinah North |
137 |
22.4 |
402 |
|
Al Batinah South |
119 |
19.5 |
266 |
|
A'Dhahirah |
67 |
11.0 |
122 |
|
A'Sharqiyah North |
43 |
7.0 |
140 |
|
Ash Sharqiyah South |
2 |
0.3 |
268 |
|
A'Dhakhiliyah |
28 |
4.6 |
246 |
|
Dhofar |
15 |
2.5 |
164 |
|
Al Buraymi |
9 |
1.5 |
62 |
|
Al Wasta |
2 |
0.3 |
20 |
*Number of patients was calculated based on the prevalence of diabetes mellitus in each governorate.
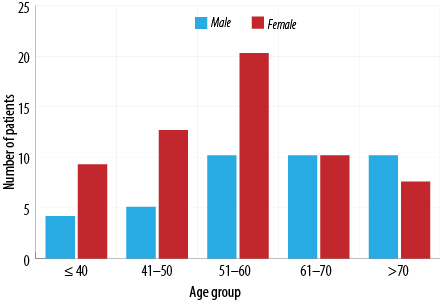
Figure 2: Age distribution among diabetic retinopathy patients in Oman.
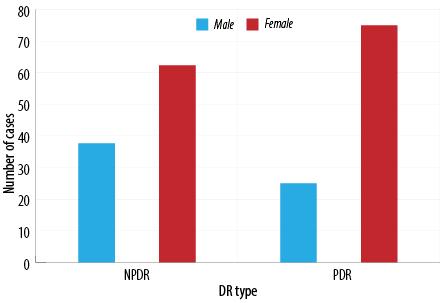
Figure 3: Distribution of diabetic retinopathy subtypes between the sexes.
Table 2: Diabetic retinopathy (DR) status based on the duration of diabetes mellitus and age > 40 years.
|
Age group, years |
≤ 40 |
> 40 |
|
0.010 |
|
Normal, n |
114 |
377 |
491 |
|
|
Retinopathy, n |
30 |
87 |
117 |
|
|
Total |
144 |
464 |
608 |
|
|
Duration, years |
< 10 |
≥ 10 |
|
0.620* |
|
Normal |
|
|
|
|
|
n |
274 |
219 |
493 |
|
|
% of DR |
55.6% |
44.4% |
|
|
|
Retinopathy |
|
|
|
|
|
n |
50 |
68 |
118 |
|
|
% of DR |
42.4% |
57.6% |
|
|
Results
Among all the 2000 patient records that were reviewed, only 616 patients met the requirements and were included in the study. Since there was such a reduction in sample size, we increased the margin of error from 2% to 5%, giving us a new sample size of 531 with 99.9% confidence level. The mean age of the population was calculated, and the gender distribution was demonstrated in Table 1. In the data file provided by the MOH, the following were the number of patients with missing data: gender - six, age - eight, and governorate - five patients. The prevalence of DR, NPDR, and PDR was shown in Figure 1. Furthermore, the distribution of each retinopathy type among the sexes and different age groups (18–29, 30–49, 50–65, and > 65 years) was also represented in Figures 2 and 3.
The mean age of the patients was 49.0 years, with an age range between 18–81 years. Table 1 shows the demographic characteristics of the patients. Females represented a higher portion of the sample than males, with 59.0% (n = 360) to 41.0% (n = 250). Moreover, Table 1 also shows the representation of each governorate within the sample collected in the study (from 616 patients). It is important to denote that the percentage representation was the highest from Muscat and the lowest were from Al Wusta and A'Sharqiya South. Musandam was excluded due to insufficient data.
Figure 1 shows that from the 616 patients included in the study, 118 were diagnosed by an ophthalmologist to have DR giving a prevalence of 19.2% (95% confidence interval (CI): 16.2–22.5). Moreover, the estimated prevalence of NPDR within the DM population was 8.6% (95% CI: 6.6–11.1) (44.9% out of those diagnosed with DR) while the prevalence of PDR was 1.3% (95% CI: 0.7–2.5) (6.8% out of those diagnosed with DR).
Females had a higher rate of 60.2% (n = 71) of DR in contrast to males with only 39.8% (n = 47). Figure 2 shows the age of patients at diagnosis of DR, the highest percentage of DR patients were diagnosed between the age of 51–60 years (n = 36) those of which are predominantly females, while the lowest rate was those ≤ 40 years of age (n = 16). There was no significant association between sex and the diagnosis of DR (p = 0.840).
Table 2 shows the age distribution in DM patients. There was no significant association between the age distribution and DR (p = 0.620). It also shows that a higher percentage of people (57.6%, n = 68) diagnosed with DR after the 10-year mark of DM (as opposed to 42.4%, n = 50 before 10 years). There was a significant association between the duration and the diagnosis of DR (p = 0.010).
Figure 3 shows the distribution of NPDR and PDR amongst gender. It was observed that in both types, females were the predominant sex with 62.3% (n = 33) diagnosed with NPDR and 75.0% (n = 6) with PDR.
Although females have the higher prevalence of each subtype, there was no significant association between PDR or NPDR and sex (p = 0.690).
Discussion
DR is one of the most devastating complications of DM and can have a major impact on patients’ quality of life. Therefore, it is crucial to detect and treat this condition in its early stages. DR is considered an indicator of systemic diabetic angiopathy and microvascular manifestations of DM. In addition, the DR status of a patient can also be used to assess the control of DM for that patient.18 There are very few studies in Oman that investigated the prevalence of DR, NPDR, and PDR. It is important to assess current health policies and produce new early intervention methods to combat this condition.
The prevalence of DR in our study was 19.2%. The DR prevalence has shown a consistent rise from 14.3% in 2003.17 This could be explained by the increasing prevalence of DM in Oman from 11.6% in 2000 to 12.3% in 2008 to 14.4% in 2017.4 The prevalence of DR in our study is considerably lower than the average DR prevalence in Asia (28%).9 A meta-analysis showed the DR prevalence of Indian (42%), South Korean (16%), Singaporean (33%), Malaysian (35%), Asian (21%), and Chinese (25%) populations.9 The prevalence in Oman falls between the South Korean and Asian populations.
Comparing Oman to other Gulf Cooperation Council countries, Kuwait and Saudi Arabia showed the highest DR prevalence of 40% and 36.8%, respectively.10,13 Oman is in line with the UAE and Bahrain with DR prevalence of 19.2%, 19%, and 20%, respectively.12,14 The variation in the prevalence might be explained by the early and annual screening program introduced for DR in Oman after the age of 40. The main issue is patient non-compliance, as many patients fail to attend annual screening sessions despite being given an appointment.
A recent local study published in May 2020 showed the prevalence of DR to be at 31%.19 This discrepancy could be explained by the fact that our study had a higher sample size and viewed patients from multiple health care centers across Oman. It is the only national study available. In addition, our study investigated the cases for almost the past 20 years. Furthermore, this study’s prevalence has a 95% confidence level, while our study has a confidence level of 99.9%.
Our study showed that women have higher DR rates, consistent with a Swedish study,20 but contradicts the previous Omani study that showed men having significantly higher rates.17 This could be explained by our study’s bigger sample size and multi-center nature. In addition, the national STEPS survey for non-communicable disease (NCD) showed that females have a higher prevalence of overweight and obesity, which has a strong correlation with DM.4 Fisher’s test was done to see whether there was an association between gender and DR, but there was no statistical significance. Other studies have not shown any difference between the
genders as well.
Our study showed a NPDR prevalence of 8.6%. This is also below the overall NPDR prevalence in Asia, which was 27%. However, the included countries showed high variability in the prevalence rate with India having the highest of 45% and South Korea having the lowest prevalence of 13%.9 A recent study conducted in the National Diabetes and Endocrine Center showed a total NPDR prevalence of 31%.19 Our study’s low prevalence could be explained by the amount of missing data in our sample, which may suggest the NPDR prevalence was under-reported.
Furthermore, our study showed a PDR prevalence of 1.3%. This was also far below the Asian average of 17%. The Asian population showed a PDR prevalence of 8%, the lowest of all the populations, while the Indian population showed the highest of all the included populations with a prevalence of 26%.9 The disparity in these statistics could also be explained by the introduction of an early screening program by the MoH, which may give a low prevalence of PDR as it is a late manifestation of DR.
In addition, our study showed that the prevalence of DR is highest in the 50–60 years age group, and the lowest prevalence was in those aged ≤ 40 years (30.5% and 13.6%, respectively). This finding is somewhat consistent with the previous study done in Oman that showed the highest prevalence between 40–49 and 50–59 years with 31.1% and 24.5%, respectively.17 This could be explained by the time needed for disease progression and that it is a late manifestation of DM. Our study investigated the relationship between the duration of DM and the patient’s DR status. There was a significant association between the duration of DM and DR consistent with previous findings.17
There was an ample amount of missing data, primarily due to referral or failure of the patients to attend the appointment. Due to the absence of a central server, we could not extract these patients’ records from their respective referral hospitals. ICD 10 coding was not used for the majority of the patients; thus, manual screening had to be done for most of the patient records. Some of the patients did not have any clinical notes entered in their records and were removed from the study.
The findings of our study suggest a need to prioritize DM prevention and control at both national and governorate levels with multisectoral, governmental, and societal support as it is an emerging threat to health, social, and economic development. In addition, there is a need for sustained public awareness campaigns and interventions to reduce the modifiable risk factors of DM, including unhealthy diet, physical inactivity, and alcohol use. There is also a need to build the health workforce’s capacity while ensuring the availability, access, affordability, and quality of safe, efficacious medicines and basic technologies for screening, diagnosing, treating, and monitoring diabetes in primary health care. Health information systems also need to be streamlined to guarantee reliable, timely, complete, and quality data for evidence-based practice and decision-making in diabetes prevention and control. Other suggestions based on our findings include promoting wellness clinics in all facilities to encourage early detection and screening of diabetes as well to serve sources of information for prevention and health promotion; strengthen implementation of Oman’s national policy for diet, physical activity, and health, and ensure continuous engagement with the agricultural sector to promote healthy diets and eating habits; introduce legislation on the production, packaging, and responsible marketing of food and drinks to reduce consumption of unhealthy foods; implementation of a physical activity tool kit in the country to encourage adoption of active lifestyles and to reduce sedentary lifestyles; and integrate NCD indicators in national health surveys to supplement the data collected for proper planning and projection of NCD prevention and control.
Conclusion
Oman employs a moderately effective screening program to combat DR. The main issue to decrease the prevalence of DR is to approach strategies in reducing the prevalence of DM, which is at a constant rise, and to increase awareness within diabetics about the importance of early screening and management of this condition. Future work regarding the national NPDR and PDR rates should be further investigated, and a larger sample size should be taken to obtain more reliable figures. These findings are important to support the formulation and implementation of DM-related policies and action plans that improve the patients’ health status.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to acknowledge the Centre of Studies and Research staff and the Ministry of Health IT department staff for their technical support.
references
- 1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006 Nov;3(11):e442.
- 2. Khandekar RB, Al-Lawati JA. Epidemiology of diabetic retinopathy in Oman: Two decades of research. Oman J Ophthalmol 2015 Jan-Apr;8(1):1-2.
- 3. International Diabetes Federation. IDF Diabetes. 8th ed. Karuranga S, editor. International Diabetes Federation. 2017 [cited 4 April 2018]. Available from: https://www.diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf.
- 4. Al-Mawali A, Jayapal SK, Morsi M, Al-Shekaili W, Pinto AD, Al-Kharusi H, et al. Prevalence of risk factors of non-communicable diseases in the Sultanate of Oman: STEPS survey 2017. Research Square; 2020 [cited date]. Available from: https://doi.org/10.21203/rs.3.rs-22519/v1.
- 5. Mahlangu D. Al. et. Annual health report 2013/2014. Gauteng Provincial Government. 2014 [cited 15 March 2018]. Available from: https://www.moh.gov.om/documents/274609/1624207/%D8%A7%D9%84%D9%81%D8%B5%D9%84+%D8%A7%D9%84%D8%AB%D8%A7%D9%85%D9%86-%D8%AA%D8%B9%D8%AF%D9%8A%D9%84/f2bbc1f0-9a7b-44ed-a35e-1ff8a7637bb2.
- 6. Zhang X, Saaddine JB, Chou C-F, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010 Aug;304(6):649-656.
- 7. Song BJ, Aiello LP, Pasquale LR. Presence and risk factors for glaucoma in patients with diabetes. Curr Diab Rep 2016 Dec;16(12):124.
- 8. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012 Mar;35(3):556-564.
- 9. Yang Q-H, Zhang Y, Zhang X-M, Li X-R. Prevalence of diabetic retinopathy, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy in Asian T2DM patients: a systematic review and Meta-analysis. Int J Ophthalmol 2019 Feb;12(2):302-311.
- 10. Al Ghamdi AH, Rabiu M, Hajar S, Yorston D, Kuper H, Polack S. Rapid assessment of avoidable blindness and diabetic retinopathy in Taif, Saudi Arabia. Br J Ophthalmol. 2012 Sep 1;96(9):1168-1172.
- 11. Al-Amer RM, Khader Y, Malas S, Abu-Yaghi N, Al-Bdour M, Ajlouni K. Prevalence and risk factors of diabetic retinopathy among Jordanian patients with type 2 diabetes. Digit J Ophthalmol DJO. 2008 Aug 4;14:42-49.
- 12. Al-Maskari F, El-Sadig M. Prevalence of diabetic retinopathy in the United Arab Emirates: a cross-sectional survey. BMC Ophthalmol 2007 Jun;7:11.
- 13. Al-Adsani AM. Risk factors for diabetic retinopathy in Kuwaiti type 2 diabetic patients. Saudi Med J 2007 Apr;28(4):579-583.
- 14. Al Alawi E, Ahmed AA. Screening for diabetic retinopathy: the first telemedicine approach in a primary care setting in Bahrain. Middle East Afr J Ophthalmol 2012 Jul-Sep;19(3):295-298.
- 15. Elshafei M, Gamra H, Khandekar R, Al Hashimi M, Pai A, Ahmed MF. Prevalence and determinants of diabetic retinopathy among persons ≥ 40 years of age with diabetes in Qatar: a community-based survey. Eur J Ophthalmol 2011 Jan-Feb;21(1):39-47.
- 16. Burgess PI, MacCormick IJ, Harding SP, Bastawrous A, Beare NA, Garner P. Epidemiology of diabetic retinopathy and maculopathy in Africa: a systematic review. Diabet Med 2013 Apr;30(4):399-412.
- 17. Khandekar R, Al Lawatii J, Mohammed AJ, Al Raisi A. Diabetic retinopathy in Oman: a hospital based study. Br J Ophthalmol 2003 Sep;87(9):1061-1064.
- 18. Maroufizadeh S, Almasi-Hashiani A, Hosseini M, Sepidarkish M, Omani Samani R. Prevalence of diabetic retinopathy in Iran: a systematic review and Meta-analysis. Int J Ophthalmol 2017 May;10(5):782-789.
- 19. Agroiya P, Alrawahi AH, Pambinezhuth F, Al Busaidi NB. Diabetic retinopathy among Omanis: Prevalence and clinical profile. Oman J Ophthalmol 2020 May;13(2):76-83.
- 20. Jerneld B, Algvere P. Relationship of duration and onset of diabetes to prevalence of diabetic retinopathy. Am J Ophthalmol 1986 Oct;102(4):431-437.