PHACES syndrome is considered a common neurocutaneous vascular disorder, and presents with a spectrum of anomalies involving different organ systems.1 It comprises posterior fossa malformations, segmental hemangioma, arterial anomalies, cardiac defects, eye anomalies, sternal cleft, or supraumbilical raphe. In 2009 a consensus diagnostic criteria for PHACES syndrome was published.2 The consensus classifies patients into PHACES or possible PHACES syndrome, based on a list of major and minor criteria used to define whether the patients qualify in the cerebrovascular, structural brain, cardiovascular, ocular, and/or ventral midline categories.2 The associated visceral hemangioma with PHACES syndrome is not considered part of the diagnostic criteria.
This report describes a unique presentation of intussusception caused by gastrointestinal (GI) hemangioma in a patient with PHACES syndrome along with a literature review looking for the association of GI hemangioma and PHACES syndrome, its common clinical manifestation, and to compare GI hemangioma with other associated visceral hemangiomas in the same series.
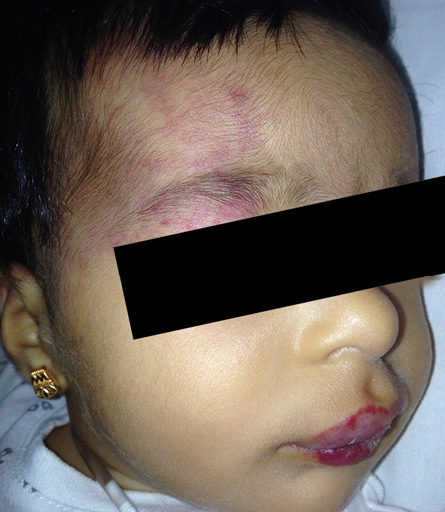
Figure 1: At presentation showing segmental telangiectatic patch with red swelling involving the right periorbital area and right forehead in addition to lips involvement.
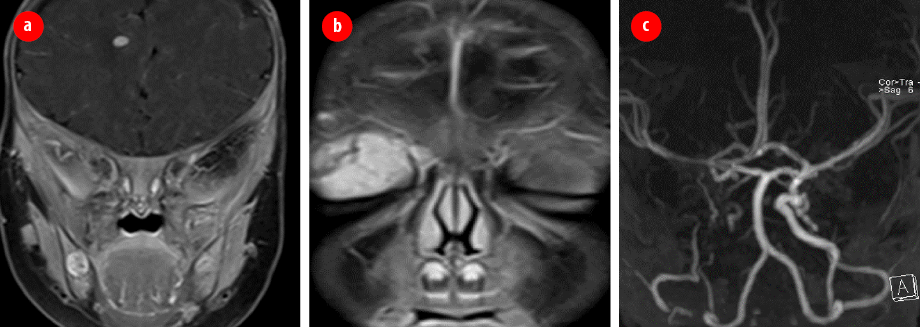
Figure 2: Brain magnetic resonance imaging and magnetic resonance angiography. (a) Mixed-signal intensity lesion in the right frontal region subcortical with features suggesting intracranial hemangioma.
(b) Intensely enhancing vascular hemangioma seen in the right preseptal region/lid extending posteriorly to the superior extra conal space. (c) Hypoplastic right internal carotid artery in its entire length to the supra-clinoid region.
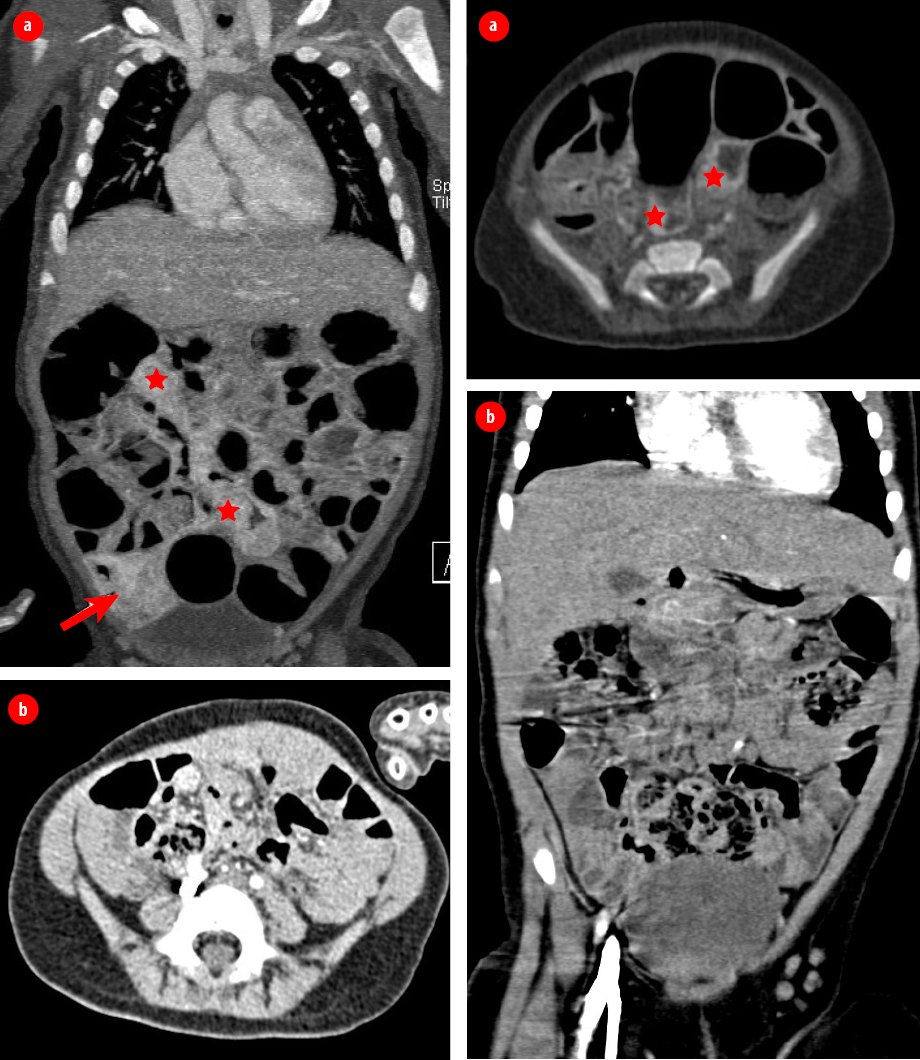
Figure 3: Computed tomography of the chest, abdomen, and pelvis with intravenous contrast in coronal and axial portal venous phase. (a) Nodular enhancement seen in the wall of different parts of the small bowel (stars) and large bowel seen at the cecum (arrow). (b) Interval reduction in the previously seen wall nodular enhancement in the small and large bowel loops after four months of treatment with propranolol.
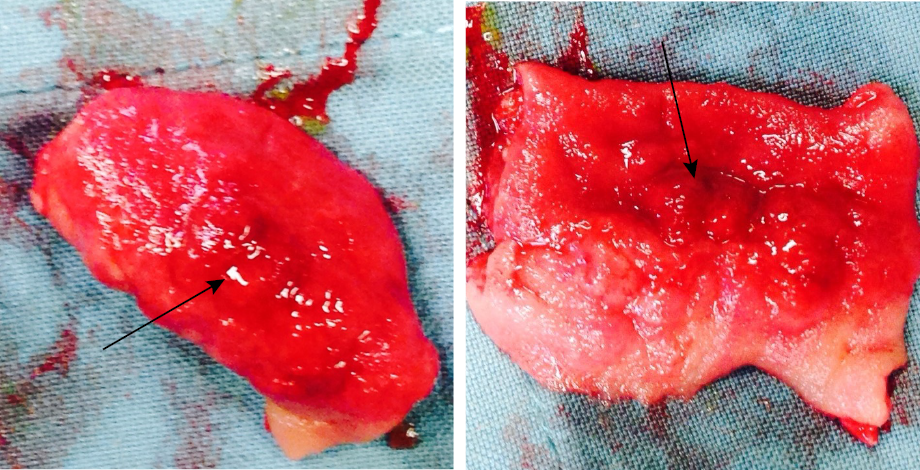
Figure 4: Intestinal hemangioma. Resected part of mid ilium showing thickened circumferential hemangioma (black arrow).
Case Report
A full-term infant girl noted at two-weeks-old with faint segmental distribution of telangiectatic patch involving the right periorbital area, right forehead, and lips [Figure 1]. At the age of three-months, she presented to the hemangioma clinic Sultan Qaboos University Hospital in Muscat, Oman, with these lesions which had progressed to more intense redness and swelling.
Due to the segmental distribution of the hemangioma over the face, PHACES syndrome was suspected. A thorough workup and evaluation, including magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), cardiology, and ophthalmology assessments were performed.
After complete workup and evaluation, the diagnosis of PHACES syndrome was confirmed based on facial segmental hemangioma, hypoplastic right internal carotid artery [Figure 2], and right optic disc anomaly. She was also found to have intracranial hemangioma. During workup, she developed projectile vomiting, abdominal pain, and redcurrant jelly stool. Ultrasound was done and showed intussusception.
Pneumatic reduction under fluoroscopy guidance was attempted three times; however, recurrence of the intussusception occurred within hours after each trial. Further evaluation by computed tomography (CT) of the chest, abdomen, and pelvis was done
[Figure 3]. Diagnostic laparoscopy revealed hemangioma covering the whole ileal wall and a thickened circumferential hemangioma covering the mid ilium was identified as the lead point of the intussusception. Laparotomy was done, and mid ilium resection of the circumferential hemangioma [Figure 4] and end-to-end anastomosis was performed. The postoperative course was uneventful. Histopathology confirmed diffuse infantile hemangioma in the resected part.
In this patient, starting propranolol was debatable given the risk of developing a stroke due to the intracranial arterial anomaly. However, considering the possible devastating consequences of proliferating hemangioma situated in critical locations like the periorbital, intestinal, and intracranial area; hence propranolol was started at a low dose (1.5 mg/kg/day) with careful monitoring. Control of this patient’s hemangiomas was ultimately achieved with propranolol 1.5 mg/kg/day and the response was evaluated by CT abdomen [Figure 3]. Propranolol was tapered off and stopped at the age of
24 months.
Discussion
We conducted a literature review to explore the relationship between GI hemangioma and PHACES syndrome [Table 1]. It is well described that segmental cutaneous infantile hemangioma can be associated with severe lower GI bleeding caused by infantile hemangioma involving a discrete segment of the GI tract.3 Visceral hemangioma is generally associated with multiple cutaneous hemangiomas. Segmental hemangiomas of the skin have a greater risk of association with structural anomalies such as those in PHACES syndrome.6 There is an additional risk of association between segmental hemangiomas of the skin and visceral hemangiomatosis, potentially causing vital organ compromise.7 Consequently, it is crucial to consider screening symptomatic patients with PHACES syndrome for the presence of visceral hemangiomatosis. Our review revealed a high prevalence of GI hemangioma compared to other visceral hemangiomas amongst the same case series of confirmed cases of PHACES syndrome [Table 2]. Metry et al,7 reported that the brain and the mediastinum are the most common sites of extracutaneous organ involvement with hemangioma in PHACES, followed by the GI tract. Since this reviewed series is limited to GI hemangioma and PHACES, it had a higher number of reported GI hemangioma than other visceral hemangioma. However, despite this limitation, it pointed to the likely association between GI hemangioma and other visceral hemangiomas in PHACES syndrome.
The most common clinical presentations of GI hemangioma are GI bleeding and anemia.3,4 Interestingly, intussusception has not been previously reported as a presentation which makes our case unique.
Table 1: The numbers and the anatomical sites of visceral hemangiomas in case series of patients with PHACES syndrome.
|
Drolet et al,3
summary of findings in nine patients with confirmed PHACES syndrome |
Liver |
3 |
|
Spleen |
1 |
|
GI tract |
9 |
|
Pancreas |
2 |
|
Paraspinal |
1 |
|
Orbital |
1 |
|
Subglottic |
2 |
|
Soukoulis et al,4
one patient with confirmed PHACES syndrome |
Neck and thorax |
3 |
|
Retroperitoneal |
1 |
|
GI tract |
1 |
|
Pascual-Castroviejo et al,5 one PHACES case |
GI tract |
1 |
|
Metry et al,1
summary of findings in 19 patients with confirmed PHACES syndrome |
Brain |
10 |
|
Mediastinum |
9 |
|
GI tract |
6 |
|
Liver |
5 |
|
Lung |
3 |
|
Pancreas |
1 |
|
Bone |
1 |
|
Brain |
1 |
Table 2: The total number and percentage of reported visceral hemangiomas classified according to the anatomical site (based on data from Table 1, n = 31).
|
Gastrointestinal tract |
18 |
58 |
|
Mediastinum + neck and thorax + lung |
15 |
48 |
|
Brain |
11 |
35 |
|
Liver |
8 |
25 |
|
Pancreas |
3 |
10 |
|
Subglottic |
2 |
6 |
|
Spleen |
1 |
3 |
|
Paraspinal |
1 |
3 |
|
Orbital |
1 |
3 |
|
Retroperitoneal |
1 |
3 |
Conclusion
There are no clear guidelines to recommend routine screening of patients with PHACES syndrome for the presence of extracutaneous hemangiomas. Future studies could possibly explore the usefulness of the addition of simple screening tests such as full blood count and fecal occult blood to the routine workup of all PHACES patients. Screening all symptomatic patients is crucial as GI hemangioma and other visceral hemangiomas are clearly associated with PHACES syndrome.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from parents.
references
- 1. Metry DW, Siegel DH, Cordisco MR, Pope E, Prendiville J, Drolet BA, et al. A comparison of disease severity among affected male versus female patients with PHACE syndrome. J Am Acad Dermatol 2008 Jan;58(1):81-87.
- 2. Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, et al; PHACE Syndrome Research Conference. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 2009 Nov;124(5):1447-1456.
- 3. Drolet BA, Pope E, Juern AM, Sato T, Howell B, Puttgen KB, et al. Gastrointestinal bleeding in infantile hemangioma: a complication of segmental, rather than multifocal, infantile hemangiomas. J Pediatr 2012 Jun;160(6):1021-1026.
- 4. Soukoulis IW, Liang MG, Fox VL, Mulliken JB, Alomari AI, Fishman SJ. Gastrointestinal infantile hemangioma: presentation and management. J Pediatr Gastroenterol Nutr 2015 Oct;61(4):415-420.
- 5. Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, García-Guereta L, López-Gutiérrez JC, Olivares P, et al. Association of cutaneous red-to-purple hemangiomas with leptomeningeal hemangiomas. a clinical study of two patients. Neuropediatrics 2010 Feb;41(1):7-11.
- 6. Winter PR, Itinteang T, Leadbitter P, Tan ST. PHACE syndrome–clinical features, aetiology and management. Acta Paediatr 2016 Feb;105(2):145-153.
- 7. Metry DW, Hawrot A, Altman C, Frieden IJ. Association of solitary, segmental hemangiomas of the skin with visceral hemangiomatosis. Arch Dermatol 2004 May;140(5):591-596.