Diabetes is a public health problem with social and financial implications for countries regardless of their economic status.1 The global prevalence of diagnosed diabetes was estimated to be 8.4% (451 million) among adults aged 18–99 years in 2017 and this is projected to increase to 9.9% (693 million) by 2045.2 Arab countries, especially Gulf Cooperation Council (GCC) states, have witnessed an unprecedented rise in the prevalence of non-communicable diseases, including diabetes.3 In Oman, a GCC-member state, the prevalence of diabetes has increased over the past three decades in parallel with rapid economic growth, urbanization, and changes in lifestyle behaviors.4 Recent estimates show that 14.5% of Omanis aged ≥ 18 years have diabetes.5 The Ministry of Health in Oman now considers diabetes to be a priority health problem.4
Diabetes can cause a myriad of complications affecting the heart, eyes, kidneys, nerves, and feet. Diabetic foot disease (DFD) — any foot pathology resulting directly from diabetes and its complications — is common, and is associated with substantial morbidity and mortality.6,7 Globally, the annual incidence of diabetic foot ulceration is 6.3%,8 and the cumulative risk of developing a foot ulcer among those with diabetes is estimated to be 19–34%.6,7 Lower limb amputation, one of the most feared and costly complications of diabetes, is at least 10–20 times more common in people with diabetes than in those without,9 and up to 75% of amputations are performed in people with diabetes.10 The five-year mortality following diabetes-related lower limb amputation is high, with reports of 40–80%.7 In Oman, around half (47.3%) of all lower limb amputations are performed in those with diabetes,11 with the annual incidence rate in this group ranging between 20–36 per 10 000.11
Implementation of preventive and therapeutic foot care measures have been shown to reduce the risk of developing foot ulceration and lower limb amputation by more than 50%.12 In Oman, diabetic foot care is mainly provided in public primary care settings and delivered by the diabetes management team, which consists of a primary care physician, a nurse, a dietitian, and a health educator.13 As part of overall diabetes care, the Omani diabetes guidelines recommend comprehensive annual foot screening for all patients with diabetes, irrespective of their foot risk status. Furthermore, it is recommended that primary care physicians refer all patients with peripheral neuropathy to a podiatrist at secondary healthcare services or to the National Diabetes and Endocrine Centre for assessment and management.13 Secondary care diabetes foot services, which include dedicated high-risk foot clinics, provide specialist assessment and management of established and severe cases of DFD. Patients with DFD that require inpatient treatment or surgical intervention are managed in tertiary regional and national hospitals.13,14
A recent study evaluated diabetes knowledge and self-management among patients with diabetes in Oman.15 Lower limb ulceration was recognized as a potential complication of diabetes by 17% of the survey participants, and only 2% identified performing foot self-care as a necessary part of their overall diabetes self-management.15 A few studies have examined patients’ adherence to regular foot self-care as part of assessing diabetes self-management,15–18 but published data on the prevalence of diabetes-related foot complications, footwear choices, and the degree to which recommended foot self-care practices are performed by patients with diabetes in Oman are lacking.11,19 Information on diabetic foot self-care practices is important for identifying gaps in the provision of diabetic foot care services,20 and developing and implementing targeted interventions aimed at improving the adoption of recommended foot care practices and reducing rates of DFD.21
Our study sought to develop and test a diabetes foot care questionnaire suitable for the Oman context. Specifically, the study aimed to explore the frequency of self-reported diabetes-related foot complications, and assess the level of foot self-care performed in an urban population with diabetes in Oman.
Methods
This was a cross-sectional study, utilizing an interviewer-administered questionnaire, conducted in A'Seeb, Muscat, Oman. There were 28 primary healthcare centers (PHCs) and two polyclinics (secondary care level) providing health care services to Omanis and non-Omanis working in the government sector in six districts throughout Muscat. Eight PHCs and one polyclinic in A'Seeb were invited to participate in the study. A'Seeb district was selected as it has the highest population density of Omani residents (total population of 310 673 in 2012, of whom 63.6% were Omani),22 the highest number of PHCs, and the highest number of registered people with diabetes compared with the other districts in Muscat. Those with diabetes in A'Seeb district represented around 29.0% of the diabetes population in Muscat.23 Furthermore, A'Seeb’s population is socioeconomically heterogeneous with different socioeconomic classes and education levels.
An interviewer-administered questionnaire was determined to be the most appropriate method for this study.24 Based on internationally recognized guidelines,24 the survey tool was developed by the research team after a detailed review of the literature. Two relevant existing questionnaires (the Diabetes Foot Care Questionnaire from the Diabetes Care Program of Nova Scotia25 and the Questionnaire for Diabetic Foot Disease26) were identified. The Diabetes Foot Care Questionnaire25 was developed to assess the risk of DFD, and the Questionnaire for Diabetic Foot Disease26 was developed and validated to facilitate the collection of community-based prevalence data for diabetes-related foot disease. Permission was obtained to utilize these questionnaires for this study. They were combined, then modified in order to better align with the local Omani culture and diabetes foot guidelines.13 The revised questionnaire covered six domains: demographic details, patient-reported diabetes-related foot disease, foot self-care, footwear choices, foot care education, and professional foot care.
The English version of the questionnaire was first pre-tested with seven individuals with diabetes recruited from a local community center in Dunedin city, New Zealand. Following pre-testing, definitions of medical terms and photos depicting foot deformities were added to the questionnaire. The questionnaire was then peer-reviewed by nine senior diabetes and family physicians with expertise in the assessment and management of diabetes and DFD, public health, and health policy. This resulted in a few minor changes related to the order and content of some questionnaire domains.
The questionnaire was then translated into Arabic by two independent medically-trained Arabic native speakers. There were no major discrepancies between each of the two translations. The Arabic version of the questionnaire was reviewed for language, clarity, and structure by a third independent expert in Arabic language with no further modifications required.
Using the same procedures used in the first pre-test, the Arabic draft of the questionnaire was then pre-tested in pilot interviews with four patients who met the study inclusion criteria attending their diabetes clinic appointments at one of the invited PHCs.
Given the exploratory nature of this study, a convenience sampling method was utilized. It was estimated that a minimum convenient sample size of 300 patients would provide sufficient information to describe the level of foot self-care practices in the study area. The sample size was increased to 350 to account for any potential missing data.
The inclusion criteria included Omanis with diabetes (type 1 or type 2) aged 20 years and over, who were registered and attended diabetes clinics at the participating primary and secondary healthcare facilities in A'Seeb. There were 3998 Omani patients who met the eligibility criteria, and the number at each of the eight participating PHCs ranged from 173 to 1000. The number of patients at each PHC invited to participate in the study was proportional to the total number of patients with diabetes registered at each respective PHC [Table 1]. Forty eligible patients were recruited from A'Seeb polyclinic.
All potential participants attending their diabetes clinic appointment were consecutively approached as they arrived for their scheduled consultation. Those who agreed to participate in the study provided informed written consent before completing the interviewer-administered questionnaire.
The interviewer-administered questionnaire was completed by the primary investigator and six research assistants (community support group members) while participants were waiting for their diabetes appointment. The research assistants received individualized and group-based training sessions on the study aims and methods, including how to administer the questionnaire. All interviews were conducted in Arabic from 3 March to 15 April 2012.
Ethical approval for the study was obtained from the National Research and Ethical Review and Approve Committee, Ministry of Health, Oman. All participants provided informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Data were entered into a pre-designed Microsoft Excel (Version 2013, Microsoft Corp., Redmond, Washington, USA). Inputted data were checked for entry errors and rechecked against the hard questionnaire copies for any other data entry errors. Where the response to a particular survey question was ‘unsure’ or the participant declined to respond, these were excluded from the analysis.
Table 1: The response rate for each participating health clinic.
|
PHC |
|
|
|
|
|
|
|
PHC1 |
206 |
5.2 |
56 |
0 |
2 |
54 (96.4) |
|
PHC2 |
695 |
17.4 |
17 |
0 |
0 |
17 (100) |
|
PHC3 |
430 |
10.8 |
44 |
1 |
0 |
43 (97.7) |
|
PHC4 |
438 |
11.0 |
37 |
0 |
0 |
37 (100) |
|
PHC5 |
563 |
14.1 |
76 |
0 |
0 |
76 (100) |
|
PHC6 |
493 |
12.3 |
34 |
0 |
0 |
34 (100) |
|
PHC7 |
173 |
4.3 |
14 |
0 |
0 |
14 (100) |
|
PHC8 |
1000 |
25.0 |
35 |
0 |
0 |
35 (100) |
|
Total |
3998 |
100 |
313 |
1 |
2 |
310 (99.0) |
|
Polyclinic |
|
|
|
|
|
|
|
APC |
NA |
NA |
40 |
0 |
0 |
40 (100) |
PHC: primary healthcare center; APC: A'Seeb polyclinic; NA: not available.
Data were expressed as proportions or means, as appropriate. A p-value of < 0.050 was considered statistically significant. All analyses were performed using STATA Statistical Software (version 10.0, STATA Corp., College Station, Texas, USA).
Foot self-care was assessed through 12 items, four of which were negatively worded (Appendix 1). Responses for each item were scored as follows: never (0), rarely (1), once a month (2), once a week (3), and daily (4). Scores were reversed for negatively worded items. For each participant, the overall foot self-care score was calculated by summing the scores for the 12 items, with a maximum score of 48 (4 points for each of the 12 items). The overall foot self-care score was divided into quartiles: scores of 37-48 were categorized as good, 25–36 as average, 13–24 as poor, and ≤ 12 as very poor.
Table 2: Sociodemographic and clinical characteristics of study participants.
|
Sex |
|
|
|
|
Male |
36.1 |
50.0 |
37.7 |
|
Female |
63.9 |
50.0 |
62.3 |
|
Age groups, yearsa |
|
|
|
|
≤ 30 |
0.6 |
2.6 |
0.9 |
|
31–39 |
8.7 |
21.1 |
10.1 |
|
40–49 |
24.6 |
21.1 |
24.2 |
|
50–59 |
31.1 |
42.1 |
32.3 |
|
60–69 |
23.6 |
5.3 |
21.6 |
|
≥ 70 |
11.3 |
7.9 |
11.0 |
|
Marital status |
|
|
|
|
Single |
2.3 |
2.5 |
2.3 |
|
Married |
80.7 |
80.0 |
80.6 |
|
Widowed |
14.5 |
12.5 |
14.3 |
|
Divorced |
2.6 |
5.0 |
2.9 |
|
Employment statusb |
|
|
|
|
Employed |
21.8 |
35.0 |
21.7 |
|
Unemployed |
64.8 |
42.5 |
57.4 |
|
Retired |
13.4 |
22.5 |
13.4 |
|
Education level |
|
|
|
|
Illiterate |
53.9 |
45.0 |
52.9 |
|
Grade 1–6 (primary) |
10.7 |
15.0 |
11.1 |
|
Grade 7–9 (intermediate) |
18.1 |
7.5 |
16.9 |
|
Grade 10–12 (secondary) |
11.6 |
27.5 |
13.4 |
|
Tertiary education |
5.8 |
5.0 |
5.7 |
|
Monthly household incomec (OR) |
|
< 300 |
49.8 |
15.6 |
46.0 |
|
300–1000 |
46.7 |
81.3 |
50.5 |
|
> 1000 |
3.5 |
3.1 |
3.4 |
|
Smoking statusd |
|
Current |
4.0 |
12.5 |
5.0 |
|
Ex-smoker |
6.0 |
5.0 |
5.9 |
|
Never |
90.0 |
82.5 |
89.1 |
|
Type of diabetese |
|
Type 1 |
7.8 |
45.0 |
12.1 |
|
Type 2 |
12.7 |
37.5 |
15.6 |
|
Do not know |
79.4 |
17.5 |
72.3 |
|
Diabetes treatment |
|
Diet alone |
7.7 |
2.5 |
7.1 |
|
Oral agents only |
74.2 |
37.5 |
70.0 |
|
Insulin |
12.6 |
42.5 |
16.0 |
|
Oral agents + insulin |
5.5 |
17.5 |
6.9 |
PC: polyclinic; PHCs: primary healthcare centers; SD: standard deviation;
OR: Omani rial.
aPHCs, n = 309, PC, n = 38; bPHCs, n = 284; cPHCs, n = 259, PC, n = 32; dPHCs, n = 300; ePHCs, n = 306; fPHCs, n = 235, PC, n = 39.
Table 3: Patient-reported diabetes-related foot disease (DRFD) signs and symptoms.
|
SPN symptoms in the last month |
|
|
|
Burning sensation in feet |
340 |
129 (38.0) |
|
Tingling in feet |
340 |
98 (28.9) |
|
Numbness in feet |
343 |
66 (19.2) |
|
Pins and needles in feet |
341 |
91 (26.7) |
|
Heaviness or tightness in feet |
338 |
92 (27.2) |
|
One or more of the SPN symptoms |
350 |
193 (55.1) |
|
PVD symptoms in the last month, |
|
|
|
Pain or cramping while walking |
|
|
|
In calves |
344 |
116 (33.7) |
|
In the back of thighs |
341 |
101 (29.6) |
|
In buttocks/bottom area |
342 |
96 (28.1) |
|
Foot pain at night |
329 |
119 (36.2) |
|
One or more of the PVD symptoms |
350 |
172 (49.1) |
|
Foot ulceration (ever) |
335 |
42 (12.5) |
|
Foot deformity signs |
|
|
|
Hammer or clawed toes |
341 |
5 (1.5) |
|
Bunions |
343 |
12 (3.5) |
|
Corns/callouses |
340 |
38 (11.2) |
|
Lumps or bumps |
336 |
36 (10.7) |
|
One or more foot deformity signs |
350 |
77 (22.0) |
SPN: sensory peripheral neuropathy; PVD: peripheral vascular disease.
Results
Of the 353 patients invited to participate in the study, one declined due to work commitments (response rate = 99.2%). Two participants were excluded from the analysis as they did not complete their questionnaire. Of the remaining 350 participants, 310 were recruited from the eight PHCs and another 40 participants from the polyclinic [Table 1].
The clinical and sociodemographic characteristics of the participants are outlined in Table 2. The duration of diabetes was longer for those patients attending the polyclinic compared with those attending the PHCs, with the overall mean duration of 7.9±7.4 years.
Table 3 describes the types of diabetes-related foot signs and symptoms as reported by the participants. During the previous month, 193 (55.1%) reported having at least one or more sensory peripheral neuropathy (SPN) symptoms, and almost half (n = 172, 49.1%) complained of one or more symptoms of peripheral vascular disease (PVD). The presence of one or more signs of foot deformity was reported by over one-fifth (22.0%) of participants, with corns/callouses being the most prevalent (11.2%). A current or previous foot ulcer was reported by 12.5% of the participants (n = 42), and 2.0% (n = 7) had had a lower limb amputation. Overall, 54 (15.4%) and 43 (12.3%) of the participants reported receiving a diagnosis of SPN and PVD, respectively.
Table 4 outlines the frequency of foot self-care practices as reported by the study participants. The foot self-care activities performed with the highest frequency daily were washing the feet (97.4%) and checking between the toes (73.5%). In contrast, fewer than half (45.3%) reported looking at the bottom of their feet daily, and one-quarter (24.9%) never inspected their shoes before wearing them. Around 17.0% of participants were unable to examine their feet, and the reasons for this included joint problems (33.3%), excess weight (41.7%), and impaired vision (11.7%).
Patient foot self-care scores are described in Figure 1. The overall mean foot self-care score was 29.0±5.8 (range = 13–43). Over two-thirds (68.0%) of participants attained an average score (25–36), while only 10.0% had a good score of 37–48.
More than half (n = 201, 57.4%) of participants reported wearing open shoes as their footwear, with traditional Omani sandals as the most common type of footwear (n = 178, 50.9%). Nearly three-quarters (72.0%) of the participants did not wear socks.
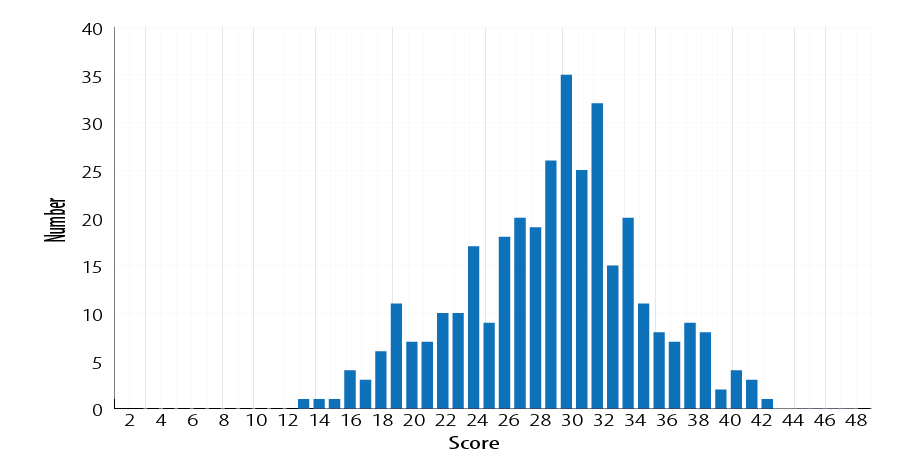
Figure 1: Foot self-care scores (n = 350).
Table 4: The reported frequency of performing the different recommended foot self-care practices.
|
Looked at bottom of feet |
344 |
13.7 |
16.6 |
11.9 |
12.5 |
45.3 |
|
Washed feet |
344 |
1.2 |
0.9 |
0.3 |
0.3 |
97.4 |
|
Checked between toes |
340 |
6.5 |
11.2 |
3.5 |
5.3 |
73.5 |
|
Dried between toes |
343 |
10.5 |
21.9 |
1.7 |
4.1 |
61.8 |
|
Tested water temperature |
337 |
20.8 |
17.8 |
1.2 |
0.9 |
59.3 |
|
Checked shoes |
342 |
24.9 |
20.2 |
1.8 |
1.2 |
52.0 |
|
Did not soak feet |
339 |
38.6 |
5.0 |
4.7 |
21.8 |
29.8 |
|
Used lubricants on feet |
340 |
10.6 |
22.4 |
0.6 |
4.7 |
61.8 |
|
Did not use lubricants between toes |
344 |
58.4 |
4.7 |
0.6 |
22.1 |
14.2 |
|
Did not walk bare foot |
345 |
50.1 |
1.2 |
0.0 |
20.6 |
28.1 |
|
Did not wear shoes without socks |
340 |
62.6 |
1.2 |
0.9 |
20.6 |
14.7 |
Note: all foot self-care practices, except for toenail cutting, are recommended to be done daily by the Omani diabetes guidelines. Toenail cutting is recommended regularly (no recommended interval).
Discussion
We explored the frequency of self-reported foot problems and foot self-care behaviors in an urban population with diabetes in Oman. Comparable with previous studies,27,28 diabetes-related foot complications were commonly reported in our population; over half (55.1%) reported symptoms of peripheral neuropathy, and 12.5% had a history of past or current foot ulceration. These results are similar to those from a similar 2011 cross-sectional study conducted in Jordanian hospitals, where 13.6% and 60.0% of surveyed participants reported a history of foot ulceration and symptoms of peripheral neuropathy, respectively.27 However, foot ulceration has been less frequently reported (0.2–5.9%) in other Arabian Gulf studies.28–30 The relatively high reported rate of foot ulceration in this study may be representative of the Omani population, but could also be explained by selection bias as a result of the convenience sampling method employed, or recall bias.
Adoption of recommended diabetes foot self-care practices by patients with diabetes is associated with a reduced risk of foot deformity, ulceration, and lower limb amputation.12 In spite of the high level of self-reported diabetes-related foot complications, recommended foot self-care behaviors were under-practiced with over two-thirds (68.0%) of participants achieving an average foot self-care score (25–36). Daily recommended foot self-care activities, such as foot self-examination (45.3%) and shoe inspection (52.0%) were infrequently performed, while undesirable and harmful behaviors, such as walking barefoot (50.1%), were practiced daily. These findings are consistent with similar local27,31,32 and international33 studies.
Local factors, cultural norms, and religious rituals may influence the adoption of evidence-based recommended foot self-care behaviors.11 It is important to note that some foot self-care practices, namely foot washing (97.4%) daily and checking between toes (73.5%) daily were performed by a relatively high proportion of our study population. This can at least be, in part, attributed to the Islamic ritual ablution (Wudhu), which is performed by Muslims three to five times per day before each prayer.27,31,32 Despite this, careful foot inspection was performed by less than half (45.3%) of participants, indicating a lack of awareness about proper foot self-care for diabetes. Further, a large proportion (50.9%) reported traditional open sandals as their preferred footwear (instead of recommended closed shoes), and 72.0% did not wear socks, a finding reported in previous local studies.27,31
The characteristically hot and dry weather in Oman can explain participants’ footwear choice since wearing the recommended closed protective footwear can be uncomfortable.11,27 Sandals do not offer the same degree of protection against external trauma afforded by closed footwear, and thus increase the risk for DFD among those with diabetes.
More than half (52.9%) of the participants were illiterate. While knowledge of diabetes is associated with the level of formal education, health literacy related to diabetes is poor among Omanis.34 The high illiteracy rate, in addition to sociocultural values, highlight the need for, and importance of, regular diabetic foot education that is simple, culturally-sensitive, and group-based.12
This study needs to be considered in light of strengths and limitations. The high response rate and the inclusion of primary and secondary care diabetes services are important strengths of the study. The absence of culturally appropriate survey tools to examine diabetic foot self-care in Oman necessitated the use of a newly-constructed interviewer-administered questionnaire, the Diabetic Foot Disease and Foot Care Questionnaire ( DFDFC-Q). The questionnaire was piloted in both New Zealand and Oman, and in the English and Arabic languages, respectively, then peer-reviewed by a group of senior diabetes and family physicians in Oman. While the validity of the DFDFC-Q was not formally evaluated, it is a culturally-sensitive tool that can be used to evaluate the effectiveness of diabetic foot educational programs. Although the methods used were thought to be appropriate to answer the objectives of this exploratory study, methodological biases (e.g., recall and interviewer biases) may have resulted in under- or over-reporting of data, such as the adherence to recommended foot self-care practices.
The design and sampling method may limit the generalizability of the findings to other areas and healthcare settings in Oman. First, the study was conducted in one urban area of the Muscat governorate, in a district that was historically known to be inhabited by a population with higher education level and socioeconomic status. Second, convenience sampling may have introduced selection bias leading to the over- or under-estimation of diabetes-related foot complications. Furthermore, data were self-reported and were not validated by reviewing participants’ medical records or undertaking an examination of their feet at the time of completing the survey.
Conclusion
Although foot problems are common in this urban Omani population with diabetes, the level of foot self-care practices was suboptimal. Findings from this study demonstrate the need for high quality, culturally-sensitive diabetic foot care education, which take into account high illiteracy levels to improve patients’ foot care awareness and self-management. Barriers to recommended foot self-care practices, such as level of foot care knowledge/education and provision of professional foot care services, need to be explored in future studies.
Disclosure
The authors declared no conflicts of interest. The study was funded by an educational scholarship from the Ministry of Higher Education, Oman. The DFDFC-Q may be reproduced and used for non-commercial research and educational purposes. Prior written permission is required from the corresponding author. Researchers must give appropriate credit and reference this article when using the DFDFC-Q.
Acknowledgements
The authors would like to express their greatest gratitude to all participating patients, health professionals, and community support group members, who provided assistance for this study. We would also like to thank the Ministry of Higher Education, Oman, for providing funding. Biostatistical advice was provided by Associate Professor Sheila Williams (Department of Preventive and Social Medicine, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand). Abstracts from this work have been presented at the 5th Annual Otago International Health Research Network Conference (Dunedin, 2012), the New Zealand Society for the Study of Diabetes Annual Conference (Queenstown, 2014), and the third Gulf Diabetic Foot Conference (Doha, Qatar, 2017).
references
- 1. Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol 2017 Jun;5(6):423-430.
- 2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018 Apr;138:271-281.
- 3. Alhyas L, McKay A, Balasanthiran A, Majeed A. Quality of type 2 diabetes management in the states of the Co-operation Council for the Arab States of the Gulf: a systematic review. PLoS One 2011;6(8):e22186.
- 4. Al-Lawati JA, Panduranga P, Al-Shaikh HA, Morsi M, Mohsin N, Khandekar RB, et al. Epidemiology of diabetes mellitus in Oman: results from two decades of research. Sultan Qaboos Univ Med J 2015 May;15(2):e226-e233.
- 5. Ministry of Health. Oman STEPS Survey fact sheet. Muscat, Oman: Ministry of Health; 2017.
- 6. Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017 Jun;376(24):2367-2375.
- 7. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005 Jan;293(2):217-228.
- 8. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017 Mar;49(2):106-116.
- 9. Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, et al. Lower extremity amputations–a review of global variability in incidence. Diabet Med 2011 Oct;28(10):1144-1153.
- 10. Narres M, Kvitkina T, Claessen H, Droste S, Schuster B, Morbach S, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: A systematic review. PLoS One 2017 Aug;12(8):e0182081.
- 11. Al-Busaidi IS, Abdulhadi NN, Coppell KJ. Care of patients with diabetic foot disease in Oman. Sultan Qaboos Univ Med J 2016 Aug;16(3):e270-e276.
- 12. van Houtum WH, Rauwerda JA, Ruwaard D, Schaper NC, Bakker K. Reduction in diabetes-related lower-extremity amputations in the Netherlands: 1991-2000. Diabetes Care 2004 May;27(5):1042-1046.
- 13. Ministry of Health. Diabetes mellitus management guidelines. 3rd ed. Ministry of Health: Oman; 2015.
- 14. Al-Busaidi IS. Diabetic foot disease in Oman: a call for more research. Oman Med J 2017 Jul;32(4):354-355.
- 15. Elliott JA, Abdulhadi NN, Al-Maniri AA, Al-Shafaee MA, Wahlström R. Diabetes self-management and education of people living with diabetes: a survey in primary health care in Muscat Oman. PLoS One 2013;8(2):e57400.
- 16. D’Souza MS, Karkada SN, Parahoo K, Venkatesaperumal R, Achora S, Cayaban AR. Self-efficacy and self-care behaviours among adults with type 2 diabetes. Appl Nurs Res 2017 Aug;36:25-32.
- 17. Alrahbi H. Diabetes self-management (DSM) in Omani with type-2 diabetes. Int J Nurs Sci 2014 Dec;1(4):352-359.
- 18. Jahan F, Al Shibli I, Mukhlif Z, Al Moqbali JA. Knowledge, attitude and barriers towards self-care practices in patients with diabetes mellitus in North Batinah, Sultanate of Oman. Int J Public Health Res 2018;6(3):63-70.
- 19. Al-Busaidi IS, Abdulhadi NN, Coppell KJ. Diabetic foot disease research in gulf cooperation council countries: a bibliometric analysis. Sultan Qaboos Univ Med J 2018 Aug;18(3):e338-e343.
- 20. Wens J, Dirven K, Mathieu C, Paulus D, Van Royen P; Belgian Diabetes Project Group. Quality indicators for type-2 diabetes care in practice guidelines: an example from six European countries. Prim Care Diabetes 2007 Feb;1(1):17-23.
- 21. van Netten JJ, Price PE, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al; International Working Group on the Diabetic Foot. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev 2016 Jan;32(Suppl 1):84-98.
- 22. National Centre of Statistics and Information. Population statistics bulletin. Oman: national centre of statistics and information. 2013. [cited 2019 March 10]. Available from: https://ncsi.gov.om/Elibrary/LibraryContentDoc/ben_Population%20statistics%20Bulletin%20No.%202_a2949de9-32b0-4252-9580-60bdd19e79bd.pdf.
- 23. Ministry of Health. Oman. Annual health report 2010, Directorate general of planning, department of information and statistics; 2010.
- 24. Boynton PM, Greenhalgh T. Selecting, designing, and developing your questionnaire. BMJ 2004 May;328(7451):1312-1315.
- 25. Diabetes care program of Nova Scotia. Foot risk assessment. 2009 [cited 2019 March 10]. Available from: https://www.yumpu.com/en/document/read/48140125/diabetes-care-program-of-nova-scotia-foot-risk-assessment-form.
- 26. Bergin SM, Brand CA, Colman PG, Campbell DA. A questionnaire for determining prevalence of diabetes related foot disease (Q-DFD): construction and validation. J Foot Ankle Res 2009 Nov;2:34.
- 27. Abu-Qamar MZ. Knowledge and practice of foot self-care among Jordanians with diabetes: an interview-based survey study. J Wound Care 2014 May;23(5):247-250, 252-254.
- 28. Al-Maskari F, El-Sadig M. Prevalence of risk factors for diabetic foot complications. BMC Fam Pract 2007 Oct;8:59.
- 29. Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One 2015 May;10(5):e0124446.
- 30. Al-Mahroos F, Al-Roomi K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: a nationwide primary care diabetes clinic-based study. Ann Saudi Med 2007 Jan-Feb;27(1):25-31.
- 31. Al Odhayani AA, Al Sayed Tayel S, Al-Madi F. Foot care practices of diabetic patients in Saudi Arabia. Saudi J Biol Sci 2017 Nov;24(7):1667-1671.
- 32. Khamseh ME, Vatankhah N, Baradaran HR. Knowledge and practice of foot care in Iranian people with type 2 diabetes. Int Wound J 2007 Dec;4(4):298-302.
- 33. Bonner T, Foster M, Spears-Lanoix E. Type 2 diabetes-related foot care knowledge and foot self-care practice interventions in the United States: a systematic review of the literature. Diabet Foot Ankle 2016 Feb;7:29758.
- 34. Al Shafaee MA, Al-Shukaili S, Rizvi SG, Al Farsi Y, Khan MA, Ganguly SS, et al. Knowledge and perceptions of diabetes in a semi-urban Omani population. BMC Public Health 2008 Jul;8:249.