Mycetoma is a chronic granulomatous skin infection and subcutaneous tissues occurs as a result of inoculation of skin with bacterial actinomycetes (actinomycetoma) or fungi (eumycetoma) through localized injury with thorns, wood fragments or insertion of hard objects. It is endemic in tropical and subtropical regions where it is a public problem with medical, social, and economic impacts. Clinically, it is characterized by a triad of painless tumefaction, draining sinuses and granules of the causative micro-organism. Identifying the type of mycetoma is vital to choose the correct therapy. The size, form, and color of granules may give the initial clues for causative organisms. The prevalence of actinomycetoma is higher than eumycetoma with greater cure rates.
Actinomycetoma is usually managed effectively with medical treatment alone. However, treatment failure can be expected and, in some patients, adding surgical intervention is necessary for decisive treatment to stop the progressing disease.
We describe a case of actinomycetoma in a 37-year-old man in the gluteal area, which was successfully treated with combined medical and surgical therapy after failure of multiple antimicrobial regimens.
Case report
A 37-year-old healthy, Omani man living in a rural area came to our skin clinic with a neglected, slowly progressing painless boggy skin lesion in his right buttock, which formed over several years [Figure 1]. He could not recall any history of trauma. The patient sought medical care in other hospitals where he was diagnosed with actinomycetoma and treated accordingly with long courses of different combined antimicrobial regimes including trimethoprim-sulfamethoxazole (SXT), aminoglycosides and amoxicillin-clavulanate. However, no clinical response was obtained.
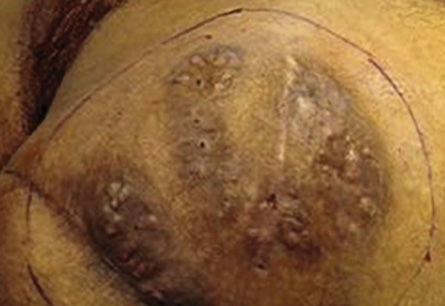
Figure 1: Right gluteal region showing the classic triad of mycetoma: tumefaction, draining sinus tracts and discharge of granules. .
Examination revealed a 15 × 12 cm painless swelling in the right gluteal area with multiple discharging sinuses. No granules were seen or regional lymphadenopathy. Histological examination of the skin biopsy revealed a clump of bacterial colony (grain) outlined by intensely eosinophilic material and surrounded by mixed inflammatory cells mostly polymorphs with granulation tissue and edema. Clusters of thin, branching filamentous actinobacteria were seen within the grain clump close to the eosinophilic material (Splendore-Hoeppli phenomenon) [Figure 2]. Culture of the specimen failed to identify the responsible organism. Basic laboratory studies were all within normal ranges. Blood tests for hepatitis B and C, HIV, and syphilis were negative. No bony involvement was detected on X-ray.
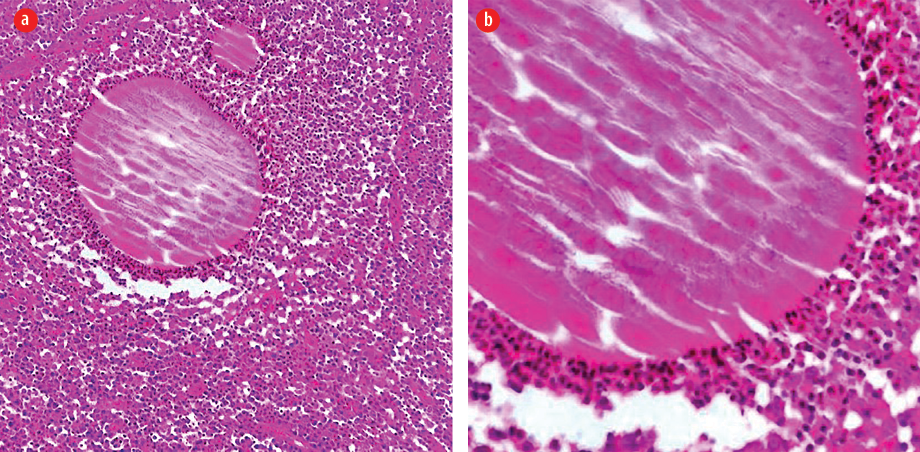
Figure 2: (a) A clump of bacterial colony (grain) outlined by intensely eosinophilic material (Splendore-Hoeppli phenomenon). The surrounding reaction is of mixed inflammatory cells mostly polymorphs with granulation tissue and edema, hematoxylin and eosin stain, magnification = × 100. (b): Clusters of thin, branching filamentous bacteria seen within the grain clump close to the eosinophilic material (Splendore-Hoeppli phenomenon), hematoxylin and eosin stain, magnification = × 400.
Initially, the patient received a combination of rifampicin 600 mg/day and dapsone 100 mg/day for nine months, which gave modest clinical improvement. Surgery was then added (excision of the affected area followed by partial thickness skin grafting) to increase the patient’s response to medical treatment [Figure 3].
Dapsone and rifampicin were continued after surgery for another six months then stopped as there was complete clinical resolution of the infection. Subsequent follow-up every three months for one year, then every six months for two years showed complete cure with no clinical recurrence [Figure 4].
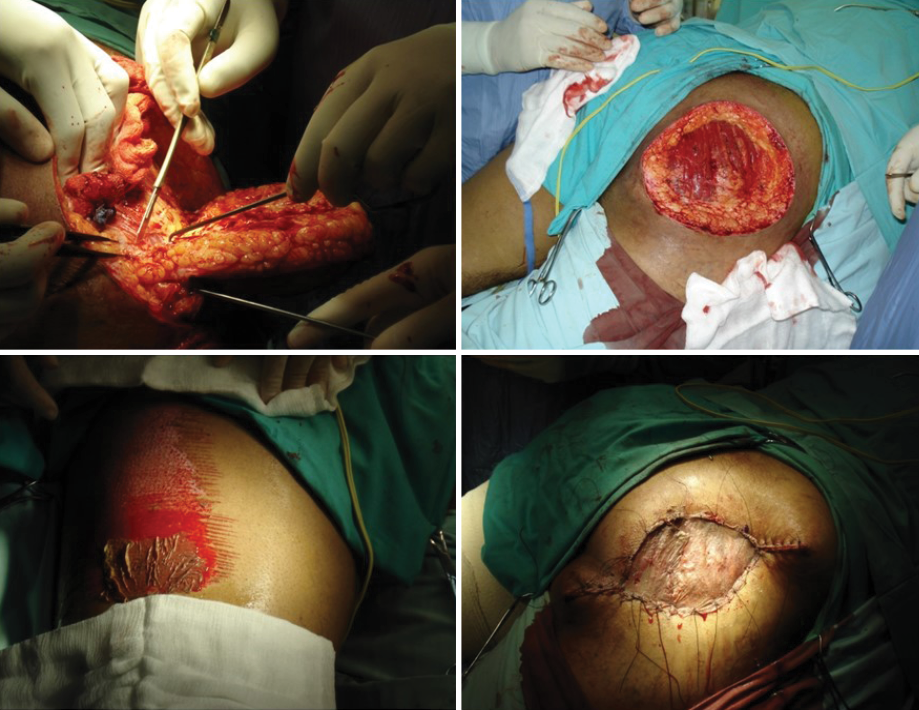
Figure 3: Excision of the affected area along with partial thickness skin graft.
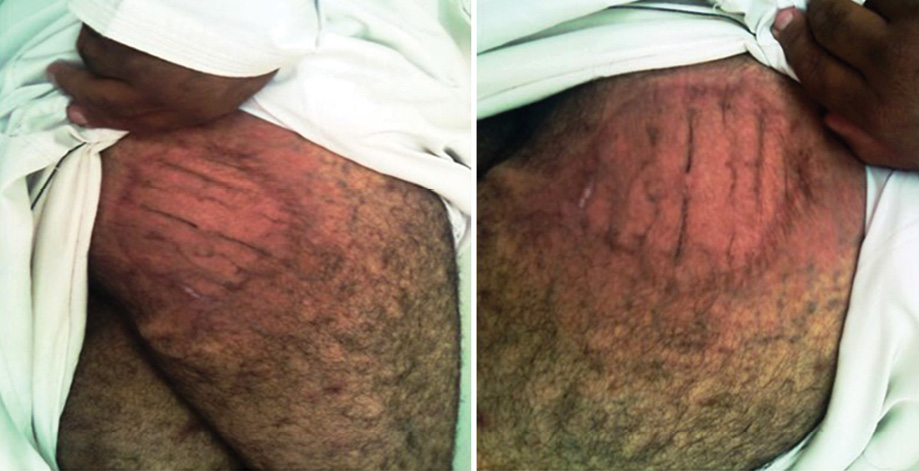
Figure 4: Complete resolution of the infection after combined medical and surgical treatment.
Discussion
Mycetoma, usually known as Madura foot, was initially described by Gill in India in 1842 and Carter used the word mycetoma for the first time in 1860.1 It is a chronic, localized, progressively destructive granulomatous inflammation of the skin and subcutaneous tissue,2–5 which can spread to involve muscles, bones and neighboring visceral organs.6,7 Two groups of mycetoma exist with similar clinical presentation; eumycetoma due to true fungi and actinomycetoma due to aerobic bacteria from actinomycomycetes species.2-5
The disease is endemic in tropical and subtropical countries including Sudan, Yemen, Senegal, India, Venezuela, Mexico, Argentina, and Colombia.1 Worldwide, 60% of mycetomas are actinomycetic in etiology.3 The most frequent causative organisms of eumycetoma are Madurella mycetomatis, M. grisea and Pseudallescheri.1 The most common organisms responsible for actinomycetoma are Actinomadura madurea, Actinomadura pelletiere, Nocardia brasiliensis, and Streptomyces somaliensis.1,8,9
Poor hygiene, inadequate nutrition and low socioeconomic status are risk factors for mycetoma.6 The responsible micro-organism lives as saprophytes in soil or plants and usually penetrates into the subcutaneous tissue of any part of the body,9 most commonly, the lower limbs of farmers and field workers who are frequently subjected to minor penetrating trauma by thorns or splinters.3 The incubation period varies from months to years, therefore patient recall of trauma is unreliable and the initial clinical presentation might be unclear for the physician and require a high index of suspicion.3,10 Reviewing the literatures showed that mycetoma is very rarely reported in Oman probably due to absence of most of the risk factors.
Both types of mycetoma present with the same clinical picture of firm tumefaction of the involved area, nodules, abscesses and sinuses that drain seropurulent exudate containing different types of granules.6,8 The diagnosis can be made based on this characteristic presentation along with direct microscopic and histopathological examinations.11
Differentiation between the two types of mycetoma is of great importance because therapy varies completely.8,11 Although it is difficult to differentiate between them clinically, actinomycetoma is more destructive, rapidly progressive and the lesions are more inflammatory than eumycetoma.2,12 The size and color of the granules seen under microscopic examination by using 10% potassium hydroxide or saline are other differentiating factors. Red grains refer to an actinomycetic origin while black or colorless grains are indicative of eumycetoma.8,11,12 White color grains are indicative of either type.9,11 Histopathological examination reveals a suppurative granulomatous reaction formed of mixed inflammatory cells, mostly neutrophils, surrounding the grains.13 Visualization of thin filaments of actinomycetic granules and thick hyphae of eumycetic granules may assist in distinguishing between the two types.12
Culture of grains to identify the micro-organisms can be taken from a deep wedge biopsy or through puncture and fine-needle aspiration.14 Unfortunately, culture of mycetoma is difficult and might not yield growth due to frequent contamination of samples by other organisms and scarcity of grains within the tissue from either previous incomplete treatment or strong immune response to the infection. In addition, the patient may present late in the disease course when fibrosis is predominant making microbiologic evaluation difficult.15
Moreover, serlological tests such as counter-immuno-electrophoresis, enzyme-linked immunosorbent assay and immunodiffusion (ID) test, are available in some centers and can be used to confirm the diagnosis and evaluate therapeutic response.8,9,13
Radiological studies including X-ray, tomography and MRI should be performed in suspected cases to determine extension to deeper tissues.8,11
Mycetoma is difficult to manage7 and treatment in early stages is vital to prevent complications and disability.11 Actinomycetomas generally respond well to antimicrobials with response rates varying from 60% to 90% compared with eumycetomas which respond poorly and tend to have a more chronic disease that require a combination of medical and surgical therapy.9
In eumycetomas, ketoconazole and itraconazole are the antifungal agents of choice,3,9,11 while in actinomycetoma trimethoprim-sulfamethoxazole (SXT) is the first line treatment, which can be used alone or in combination with other antibiotics.7–9,16 A wide range of antibiotics have been used successfully for treating actinomycetoma including aminoglycosides, rifampicin, dapsone, tetracyclines, ciprofloxacin and amoxicillin-clavulanate.7,9 Combined drug therapy is recommended to improve efficacy and avoid drug resistance. The duration for treating mycetoma is typically prolonged, particularly in cases with bony or visceral involvement, and depends on the clinical response.7 The mean treatment period is more than one year.7 Healing may be described as the absence of clinical activity with the absence of grains and negative culture.9
Surgery should be combined with appropriate antimicrobial or antifungal agents. It is indicated for localized swellings that can be totally excised. Debulking of large lesions can also increase the patient’s response to medical treatment.17 Amputation should be the last option for advanced mycetoma in extremities not responding to treatment and if associated with severe secondary bacterial infection.11
Our case report demonstrated successful treatment of a recalcitrant gluteal actinomycetoma with combined surgical excision along with antimicrobials.
Conclusion
Mycetoma is a group of chronic bacterial and fungal infectious diseases of the skin, subcutaneous tissue, and occasionally adjacent soft tissue, bone and viscera. It has a high morbidity in endemic areas. Mycetoma is rarely reported in Oman; therefore, most clinicians are not expected to be familiar with this condition which might lead to misdiagnosis particularly at the initial stage of the disease. A high index of clinical suspicion and early diagnosis can lead to eradication of the infection and prevent complications and disability. Although the vast majority of actinomycetoma is managed effectively with long-term antibiotics in different regimens, treatment failure may occur and combined medical and surgical therapy is required sometimes to reach a complete cure of the infection as reported in our case.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Rattanavong S, Vongthongchit S, Bounphamala K, Vongphakdy P, Gubler J, Mayxay M, et al. Actinomycetoma in SE Asia: the first case from Laos and a review of the literature. BMC Infect Dis 2012 Dec;12:349.
- 2. Dresch TF, Magalhase TC, Pineiro-Maceira J, Akiti T, Ramos-e-Silva M. Combined therapy for mycetoma: medical and surgical. Dermatol Surg 2010;36(6):952-954.
- 3. Maina AM, Macharia JT. Alleviating a nomad’s anguish: successful treatment of a case of leg mycetoma- a case report. Case Rep Orthop 2012;2012:753174.
- 4. Ahmed AO, van Leeuwen W, Fahal A, van de Sande W, Verbrugh H, van Belkum A. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis 2004 Sep;4(9):566-574.
- 5. Vongphoumy I, Dance DA, Dittrich S, Logan J, Davong V, Rattanavong S, et al. Case report: Actinomycetoma caused by Nocardia aobensis from Lao PDR with favourable outcome after short-term antibiotic treatment. PLoS Negl Trop Dis 2015 Apr;9(4):e0003729.
- 6. Mathews S, Jadhav R, Reza A, Karim T. Actinomycetoma-the welsh regimen in a rural Indian scenario. Indian J Surg 2012 Dec;74(6):480-482.
- 7. Ameen M, Vargas F, Arenas R, del Mercado EV. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol 2011 Apr;50(4):443-445.
- 8. Welsh O, Vera-Cabrera L, Welsh E, Salinas MC. Actinomycetoma and advances in its treatment. Clin Dermatol 2012 Jul-Aug;30(4):372-381.
- 9. Ameen M, Arenas R. Developments in the management of mycetomas. Clin Exp Dermatol 2009 Jan;34(1):1-7.
- 10. Espinoza-Gonzalez NA, Welsh O, Ocampo-Candiani J, Said-Fernandez S, Lozano-Garza G, Choi SH, et al. Evaluation of the combined therapy of DA-7218, a new oxazolidinone, and trimethoprim/ sulfamethoxazole in the treatment of experimental actinomycetoma by Nocardia brasiliensi. Curr Drug Deliv 2010 Jul;7(3):225-229.
- 11. Hjira N, Boudhas A, Al Bouzidi A, Boui M. Madura foot: report of a eumycetoma Moroccan case. Journal of dermatology and dermatologic surgery 2015;19(2):143-145.
- 12. Agarwal US, Besarwal RK, Gupta R, Agarwal P. Treatment of actinomycetoma foot–our experience with ten patients. J Eur Acad Dermatol Venereol 2013 Dec;27(12):1505-1513.
- 13. Fahal A. Evidence-based guidelines for the management of mycetoma patients. Mycetoma research center 2002:1-20.
- 14. Ayoade FO, Alam MJ. Mycetoma. [cited 2018 April 5]. Available from: www.emedicine.medscape.com.
- 15. Liu A, Maender JL, Coleman N, Hsu S, Rosen T. Actinomycetoma with negative culture: a therapeutic challenge. Dermatol Online J 2008 Apr;14(4):5.
- 16. Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH. Mycetoma medical therapy. PLoS Negl Trop Dis 2014 Oct;8(10):e3218.
- 17. Chufal SS, Thapliyal NC, Gupta MK. An approach to histology-based diagnosis and treatment of Madura foot. J Infect Dev Ctries 2012 Sep;6(9):684-688.