Bladder cancer (BC) is one of the most commonly reported cancers in the world, affecting about 550 000 people in 2018. The incidence rate in men is about four times more than that in women, and the age range of patients diagnosed is 50–54 years in both males and females, with a sharper increase in males aged 60–64 years.1 BC is the seventh most frequent cancer type worldwide. Approximately 30% of bladder tumors are anticipated to emerge from occupational exposure to carcinogens, including benzidine and 2-naphthylamine. Cigarette smoke also contains such carcinogens and is a risk factor for BC. Several jobs types, such as rubber workers, motor mechanics, leather (including shoe) workers, machine setters, bus drivers, blacksmiths, hairdressers (due to hair dye exposure), and mechanics are at greater risk of BC.2–4 The malignancy of BC in most humans appears to be multifactorial in origin and develops in multiple stages.5,6 The natural environment may consist of multiple parameters important in BC etiology.7,8 There are three main types of BC including urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma.9 Several other cell types, including stromal cells, endothelial cells, macrophages, and fibroblasts are presented within bladder tumor tissues.
Moreover, the immune system and its components play an important role in anti-tumor responses.10 On the other hand, some of the cells and mediators produced by them, such as cytokines and chemokines, can lead to tumor progression.11 Among these mediators, chemokines can modulate the development of the tumor by affecting the cancerous stromal cell types and the release of both proliferative and angiogenic factors within the tumor microenvironment (TME).12,13 Chemokines are small chemotactic cytokines and are divided into four distinct subdivisions, based on the position of the first two N-terminus cysteine residues as CC, CXC, CX3C, and XC.14–17 So far, 50 chemokines, 20 cognate chemokine receptors, and four atypical chemokine receptors (ACRs) have been discovered. Further, chemokines which are known as migratory factors in different cell types, are actively involved in cell-cell interaction and regulation of tumor proliferation, neovascularization, and metastases of the tumor.18 For instance, CXCL9, CXCL10, CXCL11 mainly contribute to the inhibition of angiogenesis and tumor progression.19 Some treatment approaches such as Bacillus Calmette-Guerin (BCG) therapy, which has been used for BC so far, can change the expression of the CXCL9, CXCL10, CXCL11 chemokines, which can play a role in the mechanisms leading to the elimination or progression of the tumor.20,21 Therefore, our review sought to assess the auxiliary role of CXCL9, CXCL10, and CXCL11 along with C-X-C motif chemokine receptor 3 (CXCR3) as their receptor in the treatment processes of BC.
1. CXCR3 biology
Cellular oriented locomotion is closely mediated through the spatial and temporal expression of chemokines.22 These small molecules regulate the cell movement, in addition to the positioning of target cells via activation of seven-transmembrane spanning G protein-coupled chemokine receptors (GPCRs).23 Chemokines are also able to bind to other non-GPCRs called ACRs. ACRs are not able to activate conventional chemokine receptor signals, but aid in maintaining chemokine gradients within the tissue.24 The differential expression pattern of chemokine receptors on leukocytes causes further selection of recruitment of specific cells leading to proper and effective immune responses in different pathologic states.25
CXCR3 is a GPCR and is also the receptor for the chemotactic factors CXCL4, CXCL9, CXCL10, and CXCL11.26 The CXCR3 gene is located on the long arm of chromosome X in region q13.27 Regarding the amino acid sequences, CXCR3 is categorized to three types including CXCR3-A, CXCR3-B, and CXCR3-alt.28 The CXCR3-A variant is the most frequent receptor expressed on the surface of immune cells, whereas CXCR3-B is expressed on the other cell types and can bind to CXCL4 along with CXCL9, CXCL10, and CXCL11, and seems to be involved in angiogenesis. CXCR3-alt is activated only by CXCL11 and known as an expressively shortened variant containing only four transmembrane helices.29 CXCR3 is not only expressed by immune cells, it is also present on resident cells like endothelial cells, vascular pericytes, and mesangial cells, and are targets for CXCL10.30 The structural–activity researches identified that if the first three residues of the CXCL11 structure are removed, chemokine retains a significant binding affinity; however, it lacks the ability for activation of CXCR3. Similarly, elimination of a few N-terminal residues of interferon-gamma (IFNγ) induces CXC chemokines CXCL9, CXCL10, and CXCL11 and results in the loss of their capacity to bind to CXCR3.31 Removal of the N-terminal from CXCL11 is physiologically relevant and dipeptidyl peptidase-4 (DPP4) successfully cleaves two residues from the N-terminal of IFN-γ inducible chemokines in vivo and relatively regulates their impacts on CXCR3.31 The duration which was determined for DPP4 cleavage is remarkably faster for CXCL11 than the period that was observed for CXCL9 and CXCL10.31 Interestingly, while all of IFN-γ inducible chemokines serve as specific ligands for CXCR3, they are also able to act as antagonists.32 T-bet promoted expression of CXCR3 on regulatory T (Treg) cells, and T-bet positive Treg cells infiltrated at sites of T helper type 1(Th1)-mediated inflammation.33 The quality of Th1 cells-based immune responses is a principal parameter for an appropriate and protective anti-tumor cellular immune response.34 Furthermore, it is well established that CXCR3-related anti-tumor responses are mediated via migration of CD4+ Th1 lymphocytes, CD8+ cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and NKT cells into the TME.35 The pivotal role for CXCR3 in the polarization of macrophage lineage was well defined recently.36 CXCR3 participated in the recruitment of CXCR3+ macrophages into the TME.37 Though, classically motivated M1 macrophages have anti-tumor activity, a wide range of tumor-associated macrophages in solid tumors are alternatively activated M2 macrophages. Evidence showed that this phenotype of macrophages (M2) could facilitate tumor development.36
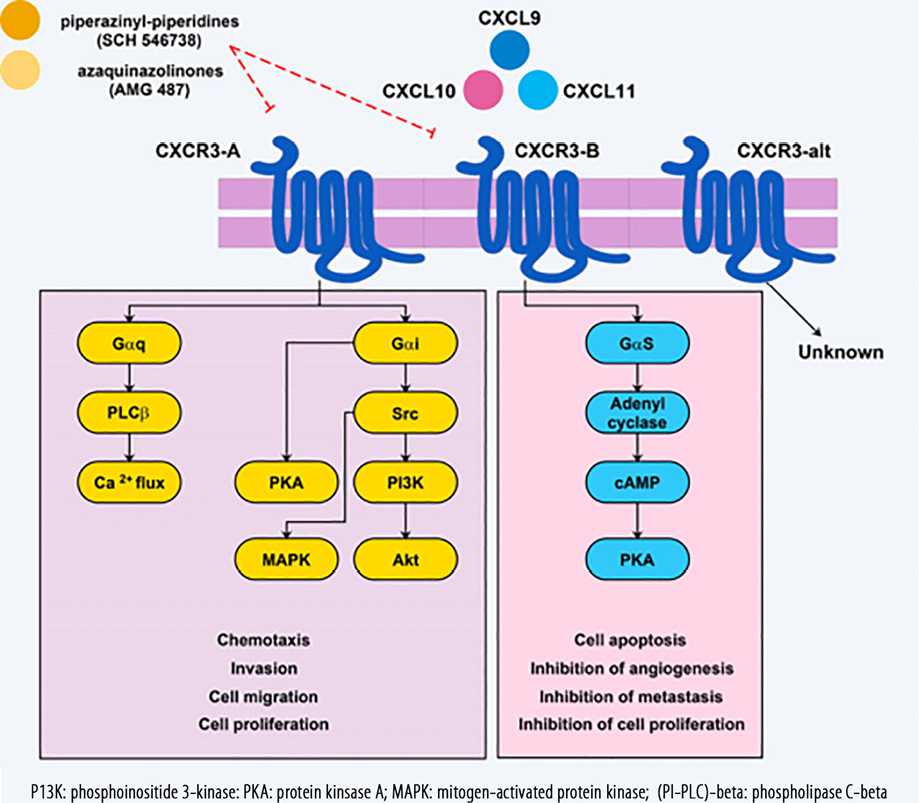
Figure 1: CXCR3 receptor subtypes and related ligands that eventually result in CXCR3-B anti-tumor mechanisms, and responses in favor of tumor progression in the CXCR3-A signaling pathway following ligation of these ligands. CXCR3 receptor antagonists that have a therapeutic effect are also identified.
2. CXCR3 signal transduction
Signaling through CXCR3-A with the assistance of a Gαi/o-protein stimulates proliferation and cell migration, while CXCR3-B signals inhibit angiogenesis, proliferation, and migration but can stimulate apoptosis [Figure 1].28 Findings of an animal model showed that knocking out Gαi2 subunits abolished chemotaxis induced by CXCR3 in lymphocytes whereas knocking out Gαi3 lead to increase attachment to GTPγS and migration of lymphocytes.38 From this study, it is understood that Gαi2 have a stimulatory function in CXCR3 signaling while Gαi3 subunits prevent signals of CXCR3 in T cells of mice. As previously noted, the CXCL10/CXCR3-B axis via protein kinase A (PKA)-phosphorylation-dependent signals is responsible for inhibition of vascular endothelial growth factor (VEGF)-induced endothelial motility, which is a necessary response for angiogenesis.39 Additionally, angiogenesis could be inhibited following ligation of CXCL4/CXCL10 to CXCR3-B and activation of the p38/mitogen-activated protein kinase (MAPK) pathway.40 Previous studies reported that in prostate cancer cell lines (DU145 and PC3), CXCL10 through µ-calpain and phospholipase C-beta(PLC-β3) stimulated cell migration and invasiveness, but normal prostate cells (RWPE-1) are involved in the reduction of cell migration through PKA-dependent signaling which block m-calpain.41 In endothelial cells, CXCR3 attachment to its ligands activates µ-calpain, causing cleavage of the cytoplasmic tail of β3 integrins and activation of caspase-3 that underlies vascular involution.42 As a final point, it was stated CXCR3-B signals through activation of p38/MAPK can mediate tumor-growth inhibition. This phenomenon is due to decreased expression of heme oxygenase-1 and by regulating Bach-1 and nuclear factor erythroid 2-related factors nuclear translocation.43,44
Immunobiology of CXCL9, CXCL10, and CXCL11 and their role in cancer
Cancer tissues are defined as complicated microenvironments consisting of several cell systems that effectively cohabit and communicate with their neighboring cells through a well-designed network.45 Events such as cell-cell interactions inside the tumor regarding the role of chemokines and cytokines as well as their impacts on immune responses and metastasis are important in understanding subsequent tumor-related mechanisms and development of the tumor.18 Cancer cells, in association with tumor-related host cells that present within TME, release a wide spectrum of chemokines leading to migration and activation of several cell types mediating the balance of anti-tumor and pro-tumor immunity.18 In addition to intrinsic cellular signals, while allowing unchecked proliferation, cancer cells can interact with their surrounding environment for forming a sustained and favorable TME. This could be achieved via promoting the phenomenon of angiogenesis, inflammation, and metastasis along with modulation of the systemic immune responses.46 Some immune cells (which are known as the effector cells including γΔ T cells, CD4+ T cells, and CTLs) are actively involved in the tumor elimination processes. They possess a unique property to either kill or control the proliferation of tumor cells.34 Moreover, these effector immune cells should migrate toward intra-tumor tissues for removal of tumor cells,47 and similarly, the presence of intra-tumor immune cells could be considered a positive prognostic indicator.
Several similar features are described for the chemokines CXCL9, CXCL10, and CXCL11 and they exhibit equality in both bio-structure and bio-function.48 IFN-γ can induce these CXC chemokines; however, the expression pattern of them is almost identical.49 Few and little differences may be related to the promoter regions of the genes encoding these chemokines,50 and with cell type-specific expression of regulatory proteins, that either selectively modulates IFN-related gene expression or bind to the regulatory sequences within the promoter of the chemokine genes. CXCL9, CXCL10, and CXCL11 also have potent anti-tumor features either by the recruitment of T lymphocytes expressing CXCR3 or through inhibiting angiogenesis.51,52 Furthermore, the anti-tumor activities of other chemokines mostly rely on the concomitant induction of these chemokines as well as the type of activated CXCR3, which can be very important in this regard [Figure 1].43,53
2.1. CXCL9
CXCL9 is known to be a monokine induced by interferon-gamma (MIG), but α and β interferons do not affect the induction of this chemokine.54 The CXCL9 gene, along with CXCL10 and CXCL11, is located on chromosome 4 and one of its most important roles is the invasion and infiltration of T lymphocytes into the tumor and inhibits tumor growth.55 Previous studies have shown that tumor cells that do not express CXCL9 exhibit more progressive manner than cells expressing CXCL9 and CXCL10.56 Compelling evidence has demonstrated that CXCL9 is expressed by multiple cancer types, such as breast,57 colorectal,58 non-small-cell lung carcinoma (NSCLC)59 and renal.60 It has been demonstrated that the overexpression of CXCL9 has led to inhibition of NSCLC tumor growth and metastasis by reduced tumor-associated angiogenesis.61 CXCL9 is a well-identified migratory factor of activated T cells and NK cells,62 which was employed as an anti-tumor therapeutic agent.63 Anti-tumor effects of CXCL9 are mediated through activation and infiltration of Th1 CD4+ cells or CTLs into the TME to eliminate the cancer.64
2.2. CXCL10
Interferon gamma-induced protein 10 is expressed by a wide variety of cells in various tissues.65 Pleiotropic effects of CXCL10 affect the immune mechanisms such as angiogenesis and organ-specific metastases of cancer. These specific remarks make CXCL10 a promising therapeutic target for a wide spectrum of disorders.66 CXCL10 was initially isolated from a human histiocytic lymphoma cell line (U937) with monocytic features and later from human placental and spleen tissues.67 The CXCL10 gene is localized on chromosome four which encodes a protein with 98 amino acids.68 In addition to four exons, its gene also contains three introns.68 An important part of the transcriptional control of the CXCL10 gene in response to IFN-γ, lipopolysaccharides, and other stimuli is mediated by a region with 230 nucleotides on the upper transcriptional start site.69 CXCL10 also serves as a chemotactic factor for T lymphocytes and attenuate their functional expansion.70 Thus, tumor infiltrated lymphocytes that were obtained from CXCL10-treated TME represent more potent cytolytic activity and produced higher levels of IFN-γ and relatively upregulated the expression of CXCL10 by cancerous cells to recruit more T cells.71 Along with CXCL9, CXCL10 has potent anti-tumor activity against tumors through recruitment of CTLs in addition to angiogenesis inhibitory properties.66
2.3. CXCL11
CXCL11 is known as an interferon-inducible T-cell alpha chemoattractant.72 The CXCL11 gene is located on human chromosome four.73 CXCL11 is predominantly expressed by leukocytes, pancreas, liver, and to a lesser extent by the thymus, spleen, lung, small intestine, placenta, and prostate.72 This chemokine is responsible for activation and accumulation of T cells.74 However, CXCL11 expression is highly responsive to IFN-γ and IFN-β but is weakly induced by IFN-α.75 Equally to other members of IFN-γ inducible CXC chemokines, CXCL11 elicits its effects on target cells through binding to CXCR3 with a higher affinity than the other ligands of CXCR3.72 It has been documented that human and mouse CXCL11 are more potent than other IFN-γ inducible chemokines such as CXCL9 and CXCL10 in engaging CXCR3+ T cells. Similarly, in vitro calcium mobilization and chemotaxis assays showed that CXCL11 is involved in phosphorylation of members of MAP kinase pathway including Akt and p44/42.76 The specific aspects of CXCL11 in activation of CXCR3 expressing cell types are strengthened by the notion that it has a unique binding region for attaching to CXCR3,77 which is the main cause for CXCR3 internalization.78 However, the anti-tumor functions of CXCL11 in vivo are yet to be clarified. The anti-tumor potential of CXCL11 was associated with intra-tumor infiltration of CD8+ and CD8+ CXCR3+ T lymphocytes and this theory could be supported by findings of investigations that showed CD8+ depletion in vivo attenuate the anti-tumor effect of CXCL11.79 This may provide evidence that the migrated CTLs in response to CXCL11 are critically involved in processes of tumor elimination.80 The murine homolog of CXCL11 was claimed to be a powerful stimulator for cytokine production and proliferation of CXCR3+ T lymphocytes in vitro.81
3. Role of CXCR3 ligands in the formation of tumor vasculature
Angiogenesis is a crucial part of progression, growth, and metastasis of cancer.82 The ELR+ CXC chemokines including CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8, which use CXCR1 and CXCR2 as their cognate receptors, are identified as angiogenesis stimulators, but ELR- ones such as CXCL4, CXCL9, CXCL10, CXCL11, and CXCL14 have shown to serve as angiostatic factors.12 Both CXCL4 and CXCL10 can suppress basic fibroblast growth factor (bFGF) and VEGF-induced angiogenesis. They can also inhibit endothelial cells proliferation and further chemotaxis.83 These effects are well evidenced to be through the ability of these chemokines to displace bFGF from heparan sulfate proteoglycan co-receptors.83 Additionally, these CXCR3 ligands recruit CD4+ Th1 cell or CD8+ CTL that are probably involved in angiostatic immune reactions. Evidence is available to show that CXCL9 can inhibit angiogenesis, and this may lead to tumor regression.84 As mentioned previously, the overexpression of CXCL9 has also been reported to be involved in the inhibition of both tumor growth and metastasis by abolishing tumor-associated angiogenesis.61 An in vitro re-stimulation experiment showed that ELR- chemokines are remarkably involved in the anti-tumor related mechanisms.85
4. Role of the CXCL9/CXCL10/CXCL11/CXCR3 axes in the treatment of BC
Chemokines and adhesion molecules play an important role in balancing pro-tumor and anti-tumor cells. Sometimes the angiogenic features of some chemokines can lead to tumor progression.86 The anti-tumoral activity of these ligands can be categorized into three main functions: 1) angiostatic effect, which leads to inhibit neovascularization of the tumor; 2) recruitment and infiltration of lymphocytes with pro-inflammatory properties to support tumor immunosurveillance; and 3) the lymphangiostatic effect, which inhibits tumor metastasis through the lymphatic vasculature.87
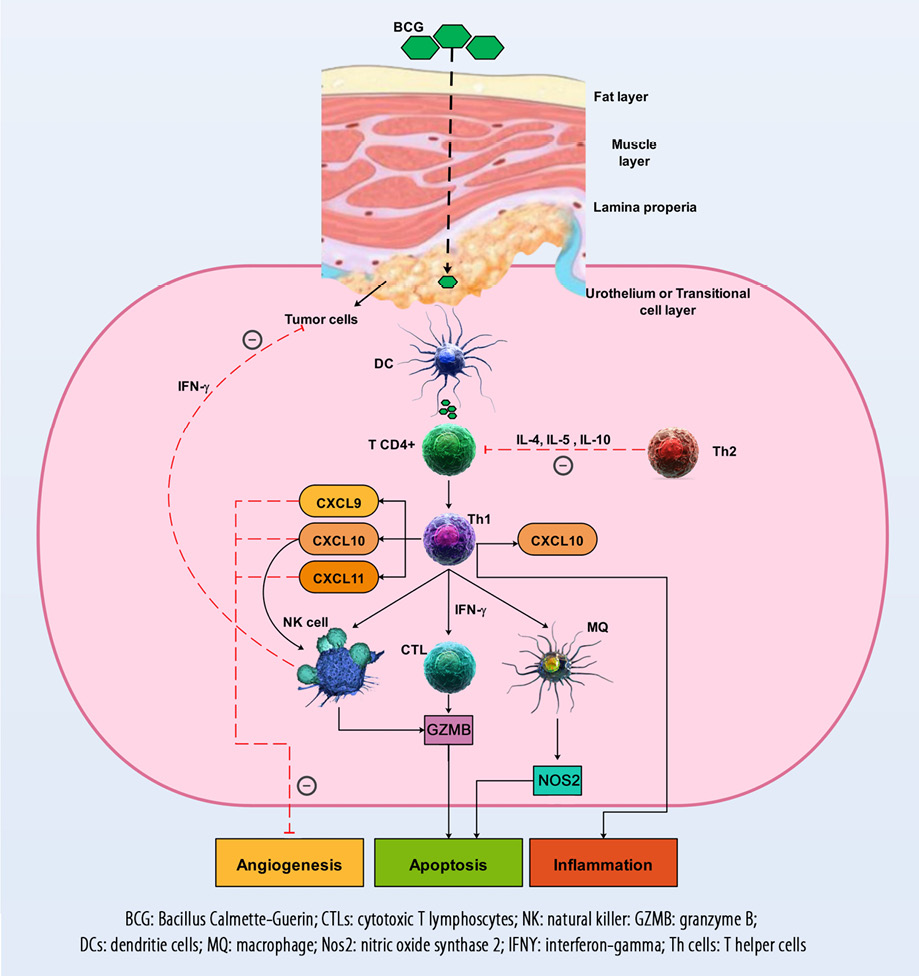
Figure 2: The immune mechanisms involved in the pathophysiology of bladder cancer as well as the responses of the CXCL9, CXCL10, CXCL11, and CXCR3 axis, which are responsible for angiogenesis inhibition, activation, and migration of immune cells such as CTLs and NK cells into the TME to prevent tumor progression. The therapeutic mechanisms and their effect on the CXCL9, CXCL10, CXCL11, and CXCR3 axis and anti-tumor immune responses are also demonstrated.
Immune cells, including CTLs, Th1, and memory cells, are recruited by the CX3CL1, CXCL9, and CXCL10 chemokines into the site of tumor [Figure 2].88 After tumor implantation in mice (day 7), gene expression of CXCL10 was significantly increased while CXCL11 expression increased on day 28. The authors of the study suggested that therapeutic approaches, including induction of Th1 and inhibition of Th2 might improve immune responses in BC therapy.89 Another study showed that after intravesical BCG instillation, CXCL10 was the main CXC chemokine detected in the urine of patients with BC. In addition, this study demonstrated that the expression of CXCL10 was considerably elevated in T24 cells.90 In the human BC cell line (RT4), CXCL10 levels significantly increased and were shown to be involved in the balance of Th1/Th2 responses.91
The survival rate in 15–20% of patients suffering from all stages of urothelial cancers (carcinomas of the bladder, ureters, and renal pelvis) is approximately five years.92 Since 2015, programmed cell death protein and programmed death ligand 1 immune checkpoint therapies are used for urothelial cancers.93 Comparing median overall survival and objective response rates with historical controls, nivolumab, atezolizumab, avelumab, and durvalumab were approved based on single-arm investigations, and the only approved therapy in a randomized phase III trial is pembrolizumab.92 However, these types of monoclonal antibody-based therapies affect immune checkpoints and have no effect on increasing the expression of CXCL9, CXCL10, and CXCL11 chemokines to recruit T cells into the TME. Given the importance of the CXCL9, CXCL10, and CXCL11 chemokines and their anti-tumor properties, we focus on BC therapies that are related or increase the expression of these chemokines.
4.1. BCG therapy
For more than a quarter-century, BCG has been used to treat non-muscle-invasive BC.94 BCG has been used as one of the best biotherapies in the treatment of cancer, but despite numerous clinical experiences in this field, its mechanisms of action have not yet been elucidated.20 Evidence proposes that urothelial cells and immune cells are generally involved in the therapeutic effects of BCG. Tumor cells may be involved through the binding and internalization of BCG, as well as the secretion of cytokines and chemokines, and the presentation of BCG and tumor antigens to immune cells. Macrophages, lymphocytes CD4+ and CD8+, granulocytes, NK cells, and dendritic cells as immune system components can kill BC cells directly via the production of soluble factors such as tumor necrosis factor-related apoptosis-inducing ligand. Also, BCG, to some extent, can directly kill tumor cells [Figure 2].20
Both anti-tumor response and immune system activation appear to be deserved for BCG live, in addition to its closed contact with tumor cells. Previous evidence revealed that viable BCG was required for therapeutic efficacy.95 Studies clearly showed that BC tissues are unable to generate sufficient levels of CXCL10 within the first week of BCG therapy. However, the potency of CXCL10 production is restored after three weekly dosages of BCG.21,96 The combination of IFNα and poly-I:C (a TLR3 ligand) or BCG + IFNα + poly-I:C (although neither BCG + IFNα nor BCG + poly-I:C) is thought to be highly beneficial for enhancement of intra-tumor production of CXCL10 and CTLs infiltration in BC tissues.96 These effective combinations have been demonstrated in particular to selectively induce CXCL10 (rather than CCL22) in cancer tissues.96 BCG has also remarkably upregulated genes involved in the production of Th1 chemokines, such as CXCL2, CXCL9, CXCL10, CXCL11, and most of the genes of these chemokines remained overexpressed after six weeks.97 Another study revealed that CXCL10 and its upstream regulatory anti-angiogenic cytokines, including (IFN-γ and interleukin-12), were significantly elevated during intra-vesicular BCG immunotherapy of BC.98 The urinary measures of IFN-γ, CXCL10, tumor necrosis factor alpha, and vascular cell adhesion molecule 1 were increased in BC patients under treatment with BCG.99 This concept proposes that intra-vesicular BCG induces a cytokine-rich urinary microenvironment that serves as an inhibitory factor against human endothelial cells with angiostatic properties.99 Some BCs are involved in the production of the Th2 macrophage-derived chemokines, which may alienate the BCG-induced Th1 cell in TME and may be a potential cause for BCG non-responsiveness.100 Primary TCC cells and endothelial cell lines also produce CXCL10 in response to BCG or IFN treatment in vitro. Another study detected an alteration in expression of a gene profile related to chemokines, which occurred after BCG administration in the bladders of healthy mice.97 They also observed that BCG has remarkably upregulated expression of CXCL9 and CXCL10 genes after four weeks and that approximately all of these genes were overexpressed after six weeks.97 Moreover, other recent studies confirmed that in response to live BCG, T cells migrate to the bladder tissue while heat-killed BCG failed to do so.101 Animal-based studies revealed that to be effective therapy, BCG must be administered intralesionally.102 This model is also true for urothelial cancer and lesional regions that are not accessible to intravesical BCG, including the upper urinary tract or prostatic ducts. Multiple studies have revealed that on subsequent intravesical BCG therapy, there is vigorous stimulation of pro-inflammatory chemokines such as CXCL10.21,98,103 The production of CXCL8 significantly improved the capacity of BCG to induce the production of CXCL10 by DCs. This phenomenon indicates positive interactions between Th1 cells and DCs.104 Therefore, it can be concluded that in BC patients, BCG treatment can help in various ways, such as increasing the expression of CXCL9, CXCL10, and CXCL11 chemokines.
4.2. CXCR3 antagonists
As already mentioned, the CXCR3 type and related activated signaling pathway can act as a double-edged sword in tumor-related immune responses.105 The activation of CXCR3-B can prevent progression of the tumor, while the activation of the CXCR3-A will cause tumor development.87 Hence, inhibiting this receptor with its antagonists can sometimes help treatment of the tumor. So far, more than 15 types of CXCR3 receptor antagonists have been identified. Two of the most prominent antagonists include piperazinyl-piperidines (SCH 546738) and aza-quinazolinones (AMG 487).106 Existing evidence suggests that piperazinyl-piperidines have been studied more in experimental models.106,107 While aza-quinazolinones are the only known antagonist for CXCR3, which has been evaluated in clinical trials.108 There is no study that these small molecules have been used in the treatment of BC. One of the major problems reported in using these antagonists is the attachment of these small molecules to different and non-specific locations within the receptor’s structure.109,110 Hence, further studies are required to extend the use of these antagonists and the production of new molecules with specific binding capabilities to target sites in different receptors of the chemokines.
Conclusion
The recruitment of T cells into the TME by CXCL9, CXCL10, and CXCL11 is important for the removal of tumors in patients with BC. Treatments such as the use of BCG (due to its effect on increasing the expression of CXCL9, CXCL10, and CXCL11 chemokines) can lead to infiltration of the T cells in the TME and eliminate the tumor. Moreover, in some cases, when the CXCR3 and its downstream signals are angiogenic, it may be possible to use the receptor blockers to prevent the angiogenesis and progression of the tumor. However, the impact of various treatments on BC and their effect on the expression of CXCL9, CXCL10, and CXCL11 requires further studies.
Disclosure
The authors declared no conflict of interest. This project was supported by Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
references
- 1. Inamura K. Bladder Cancer: New Insights into Its Molecular Pathology. Cancers (Basel) 2018 Apr;10(4):100.
- 2. Reulen RC, Kellen E, Buntinx F, Brinkman M, Zeegers MP. A meta-analysis on the association between bladder cancer and occupation. Scandinavian journal of urology and nephrology 2008;42(sup218):64-78.
- 3. Cumberbatch MG, Cox A, Teare D, Catto JW. Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol 2015 Dec;1(9):1282-1290.
- 4. Nazari A, Khorramdelazad H, Hassanshahi G. Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer. Int J Clin Oncol 2017 Dec;22(6):991-1000.
- 5. Oyasu R, Hopp ML. The etiology of cancer of the bladder. Surg Gynecol Obstet 1974 Jan;138(1):97-108.
- 6. Lower GM Jr. Concepts in causality: chemically induced human urinary bladder cancer. Cancer 1982 Mar;49(5):1056-1066.
- 7. Morrison AS, Cole P, Maclure K. Epidemiology of urologic cancers. Javadpour N, editor. In: principles and management of urologic can-cer. Williams and Wilkins Press, Baltimore; 1979. p. 1-27.
- 8. Lower G Jr, Bryan G. Etiology and carcinogenesis: natural systems approaches to causality and control. Javadpour N, editor. In: Principles and management of urologic cancer. Williams and Wilkins Press, Baltimore; 1979. p. 29-53.
- 9. Kantor AF, Hartge P, Hoover RN, Fraumeni JF. Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer research 1988;48(13):3853-3855.
- 10. Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci 2018 Mar;109(3):523-530.
- 11. Liang K, Liu Y, Eer D, Liu J, Yang F, Hu K. High CXC chemokine ligand 16 (CXCL16) expression promotes proliferation and metastasis of lung cancer via regulating the NF-κB pathway. Med Sci Monit 2018 Jan;24:405-411.
- 12. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res 2014 Dec;2(12):1125-1131.
- 13. Wang C, Li A, Yang S, Qiao R, Zhu X, Zhang J. CXCL5 promotes mitomycin C resistance in non-muscle invasive bladder cancer by activating EMT and NF-κB pathway. Biochem Biophys Res Commun 2018 Apr;498(4):862-868.
- 14. Karimabad MN, Khoramdelazad H, Hassanshahi G. Genetic variation, biological structure, sources, and fundamental parts played by CXCL12 in pathophysiology of type 1 diabetes mellitus. Int J Diabetes Dev Ctries 2017;37(3):229-239.
- 15. Nazari, A, Sardoo AM, Fard ET, Khorramdelazad H, Hassanshahi G, Nadimi AE. Plasma CXCL16 level is associated with cardiovascular disease in Iranian hemodialysis patients. Biomedical and Pharmacology Journal 2017;10(1):01-07.
- 16. Nazari A, Hassanshahi G, Khorramdelazad H. Elevated levels of epithelial neutrophil activating peptide-78 (ENA-78)(CXCL5) and Interleukin-1β is correlated with varicocele-caused infertility: A novel finding. Middle East Fertil Soc J 2017;22(4):333-335.
- 17. Behfar S, Hassanshahi G, Nazari A, Khorramdelazad H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine 2018;110:226-231.
- 18. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004 Jul;4(7):540-550.
- 19. Kistner L, Doll D, Holtorf A, Nitsche U, Janssen KP. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget 2017 Sep;8(52):89998-90012.
- 20. Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 2014 Mar;11(3):153-162.
- 21. Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol 2009 Apr;181(4):1571-1580.
- 22. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659-702.
- 23. Ziarek JJ, Kleist AB, London N, Raveh B, Montpas N, Bonneterre J, et al. Structural basis for chemokine recognition by a G protein-coupled receptor and implications for receptor activation. Sci Signal 2017 Mar;10(471):eaah5756.
- 24. Ulvmar MH, Hub E, Rot A. Atypical chemokine receptors. Exp Cell Res 2011 Mar;317(5):556-568.
- 25. Yoshie O, Matsushima K. Chemokines and Chemotaxis. Inflammation: From Molecular and Cellular Mechanisms to the Clinic; 2018. p. 619-650.
- 26. Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, et al. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem 1998 Jul;273(29):18288-18291.
- 27. Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol 1998 Nov;28(11):3696-3705.
- 28. Ma B, Khazali A, Wells A. CXCR3 in carcinoma progression. Histol Histopathol 2015 Jul;30(7):781-792.
- 29. Andrews SP, Cox RJ. Small molecule CXCR3 antagonists. J Med Chem 2016 Apr;59(7):2894-2917.
- 30. Arimilli S, Ferlin W, Solvason N, Deshpande S, Howard M, Mocci S. Chemokines in autoimmune diseases. Immunol Rev 2000 Oct;177(1):43-51.
- 31. Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 2001 Dec;98(13):3554-3561.
- 32. Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem 2001 Feb;276(5):2986-2991.
- 33. Koch MA, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation 2009;10(6):595.
- 34. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-271.
- 35. Andersson A, Yang SC, Huang M, Zhu L, Kar UK, Batra RK, et al. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol 2009 Jun;182(11):6951-6958.
- 36. Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, et al. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology 2014 Sep;143(1):109-119.
- 37. Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med 1993 Sep;178(3):1057-1065.
- 38. Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, et al. Inhibition of G α i2 activation by G α i3 in CXCR3-mediated signaling. J Biol Chem 2007 Mar;282(13):9547-9555.
- 39. Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 2006 Mar;98(5):617-625.
- 40. Petrai I, Rombouts K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG, et al. Activation of p38(MAPK) mediates the angiostatic effect of the chemokine receptor CXCR3-B. Int J Biochem Cell Biol 2008;40(9):1764-1774.
- 41. Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer 2012 Jan;11(1):3.
- 42. Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. IP-10 induces dissociation of newly formed blood vessels. Journal of cell science 2009;122(12):2064-2077.
- 43. Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S. CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J Biol Chem 2010 Nov;285(47):36842-36848.
- 44. Balan M, Pal S. A novel CXCR3-B chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation. J Biol Chem 2014 Feb;289(6):3126-3137.
- 45. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008 Oct;27(45):5904-5912.
- 46. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006 Jan;6(1):24-37.
- 47. Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999 Apr;5(4):405-411.
- 48. Tensen CP, Flier J, Van Der Raaij-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, et al. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J Invest Dermatol 1999 May;112(5):716-722.
- 49. Xanthou G, Duchesnes CE, Williams TJ, Pease JE. CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. Eur J Immunol 2003 Aug;33(8):2241-2250.
- 50. Tensen CP, Flier J, Rampersad SS, Sampat-Sardjoepersad S, Scheper RJ, Boorsma DM, et al. Genomic organization, sequence and transcriptional regulation of the human CXCL 111 gene. Biochimica et Biophysica Acta 1999;1446(1-2):167-172.
- 51. Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, et al. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol 1998 Sep;64(3):384-392.
- 52. Paparo SR, Fallahi P. Bladder cancer and Th1 chemokines. Clin Ter 2017 Jan-Feb;168(1):e59-e63.
- 53. Sharma S, Yang SC, Hillinger S, Zhu LX, Huang M, Batra RK, et al. SLC/CCL21-mediated anti-tumor responses require IFNgamma, MIG/CXCL9 and IP-10/CXCL10. Mol Cancer 2003 Apr;2(1):22.
- 54. Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997 Mar;61(3):246-257.
- 55. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev 2018 Feb;63:40-47.
- 56. Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, et al. CXC chemokine ligand 9/monokine induced by IFN-γ production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol 2007 Feb;178(4):2278-2286.
- 57. Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stöckel A, Avril S, et al. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res 2012 Feb;14(1):R30.
- 58. Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol 2004 Nov;173(10):6234-6240.
- 59. Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS, et al. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res 2001 Dec;61(23):8498-8503.
- 60. Dias S, Choy M, Rafii S. The role of CXC chemokines in the regulation of tumor angiogenesis. Cancer Invest 2001;19(7):732-738.
- 61. Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, et al. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther 2000 Jan;11(2):247-261.
- 62. Hertenstein A, Schumacher T, Litzenburger U, Opitz CA, Falk CS, Serafini T, et al. Suppression of human CD4+ T cell activation by 3,4-dimethoxycinnamonyl-anthranilic acid (tranilast) is mediated by CXCL9 and CXCL10. Biochem Pharmacol 2011 Sep;82(6):632-641.
- 63. Zhang R, Tian L, Chen LJ, Xiao F, Hou JM, Zhao X, et al. Combination of MIG (CXCL9) chemokine gene therapy with low-dose cisplatin improves therapeutic efficacy against murine carcinoma. Gene Ther 2006 Sep;13(17):1263-1271.
- 64. Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev 2006 Sep;25(3):357-371.
- 65. Ahmadi Z, Arababadi MK, Hassanshahi G. CXCL10 activities, biological structure, and source along with its significant role played in pathophysiology of type I diabetes mellitus. Inflammation 2013 Apr;36(2):364-371.
- 66. Karin N, Razon H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018 Sep;109:24-28.
- 67. Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998 Feb;338(7):436-445.
- 68. Ohmori Y, Hamilton TA. Cell type and stimulus specific regulation of chemokine gene expression. Biochem Biophys Res Commun 1994 Jan;198(2):590-596.
- 69. Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem 1993 Mar;268(9):6677-6688.
- 70. Padovan E, Spagnoli GC, Ferrantini M, Heberer M. IFN-α2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol 2002 Apr;71(4):669-676.
- 71. Yang X, Chu Y, Wang Y, Zhang R, Xiong S. Targeted in vivo expression of IFN-γ-inducible protein 10 induces specific antitumor activity. J Leukoc Biol 2006 Dec;80(6):1434-1444.
- 72. Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 1998 Jun;187(12):2009-2021.
- 73. Erdel M, Laich A, Utermann G, Werner ER, Werner-Felmayer G. The human gene encoding SCYB9B, a putative novel CXC chemokine, maps to human chromosome 4q21 like the closely related genes for MIG (SCYB9) and INP10 (SCYB10). Cytogenet Cell Genet 1998;81(3-4):271-272.
- 74. Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol 2018 Apr;51:140-145.
- 75. Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM. Characterization of β-R1, a gene that is selectively induced by interferon β (IFN-β) compared with IFN-α. J Biol Chem 1996 Sep;271(37):22878-22884.
- 76. Meyer M, Hensbergen PJ, van der Raaij-Helmer EM, Brandacher G, Margreiter R, Heufler C, et al. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol 2001 Aug;31(8):2521-2527.
- 77. Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, et al. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell α chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol 2001 Apr;59(4):707-715.
- 78. Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell α chemoattractant (CXCL11). J Immunol 2001 Dec;167(12):7084-7093.
- 79. Wang W, Green M, Liu JR, Lawrence TS, Zou W. CD8+ T Cells in Immunotherapy, Radiotherapy, and Chemotherapy. Springer, Cham; In Oncoimmunology 2018. p. 23-39.
- 80. Kakimi K, Matsushita H, Hosoi A, Miyai M, Ohara O. CTLs regulate tumor growth via cytostatic effects rather than cytotoxicity: a few T cells can influence the growth of many times more tumor cells. Oncoimmunology 2014 Dec;4(3):e970464.
- 81. Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, et al. Chemokine monokine induced by IFN-γ/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol 2004 Jun;172(12):7417-7424.
- 82. Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. InAdvances in cancer research 2010;106:91-111.
- 83. Perollet C, Han ZC, Savona C, Caen JP, Bikfalvi A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood 1998 May;91(9):3289-3299.
- 84. Huang Y, Kim BY, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol 2018 Mar;18(3):195-203.
- 85. Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother 2005 Jul-Aug;28(4):343-351.
- 86. Mukaida N, Sasaki , and T. Baba. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators of inflammation 2014;2014.
- 87. Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev 2015 Jun;26(3):311-327.
- 88. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012 Mar;12(4):298-306.
- 89. Tham SM, Ng KH, Pook SH, Esuvaranathan K, Mahendran R. Tumor and microenvironment modification during progression of murine orthotopic bladder cancer. Clinical and Developmental Immunology 2011;2011.
- 90. Luo Y, Chen X, O’Donnell MA. Mycobacterium bovis bacillus Calmette-Guérin (BCG) induces human CC- and CXC-chemokines in vitro and in vivo. Clin Exp Immunol 2007 Feb;147(2):370-378.
- 91. Yamada H, Odonnell MA, Matsumoto T, Luo Y. Interferon-γ up-regulates toll-like receptor 4 and cooperates with lipopolysaccharide to produce macrophage-derived chemokine and interferon-γ inducible protein-10 in human bladder cancer cell line RT4. J Urol 2005 Sep;174(3):1119-1123.
- 92. Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. Journal for immunotherapy of cancer 2017;5(1):94.
- 93. Ghasemzadeh A, Bivalacqua TJ, Hahn NM, Drake CG. New strategies in bladder cancer: a second coming for immunotherapy. Clinical Cancer Research 2016;22(4):793-801.
- 94. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976 Aug;116(2):180-183.
- 95. Kelley DR, Ratliff TL, Catalona WJ, Shapiro A, Lage JM, Bauer WC, et al. Intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: effect of bacillus Calmette-Guerin viability on treatment results. J Urol 1985 Jul;134(1):48-53.
- 96. Muthuswamy R, Wang L, Pitteroff J, Gingrich JR, Kalinski P. Combination of IFNα and poly-I:C reprograms bladder cancer microenvironment for enhanced CTL attraction. J Immunother Cancer 2015 Mar;3(1):6.
- 97. Seow SW, Rahmat JN, Bay BH, Lee YK, Mahendran R. Expression of chemokine/cytokine genes and immune cell recruitment following the instillation of Mycobacterium bovis, bacillus Calmette-Guérin or Lactobacillus rhamnosus strain GG in the healthy murine bladder. Immunology 2008 Jul;124(3):419-427.
- 98. Poppas DP, Pavlovich CP, Folkman J, Voest EE, Chen X, Luster AD, et al. Intravesical bacille Calmette-Guérin induces the antiangiogenic chemokine interferon-inducible protein 10. Urology 1998 Aug;52(2):268-275, discussion 275-276.
- 99. Pavlovich CP, Kräling BM, Stewart RJ, Chen X, Bochner BH, Luster AD, et al. BCG-induced urinary cytokines inhibit microvascular endothelial cell proliferation. J Urol 2000 Jun;163(6):2014-2021.
- 100. Yamada H, Luo Y, Matsumoto T, O’Donnell MA. A novel expression of macrophage derived chemokine in human bladder cancer. J Urol 2005 Mar;173(3):990-995.
- 101. Biot C. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012;4(137):137ra72.
- 102. Zbar B, Bernstein ID, Bartlett GL, Hanna Jr MG, Rapp HJ. Immunotherapy of cancer: regression of intradermal tumors and prevention of growth of lymph node metastases after intralesional injection of living Mycobacterium bovis. Journal of the National Cancer Institute, 1972;49(1):119-130.
- 103. Casrouge A, Bisiaux A, Stephen L, Schmolz M, Mapes J, Pfister C, et al. Discrimination of agonist and antagonist forms of CXCL10 in biological samples. Clin Exp Immunol 2012 Jan;167(1):137-148.
- 104. Szpakowski P, Biet F, Locht C, Paszkiewicz M, Rudnicka W, Druszczyńska M, et al. Dendritic cell activity driven by recombinant Mycobacterium bovis BCG producing human IL-18, in healthy BCG vaccinated adults. Journal of immunology research 2015;2015.
- 105. Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2013;1836(2):287-295.
- 106. Jenh C-H, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC, et al. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 2012 Jan;13(1):1-4.
- 107. Poulet FM, Penraat K, Collins N, Evans E, Thackaberry E, Manfra D, et al. Drug-induced hemolytic anemia and thrombocytopenia associated with alterations of cell membrane lipids and acanthocyte formation. Toxicol Pathol 2010 Oct;38(6):907-922.
- 108. Berry K, Friedrich M, Kersey K, Stempien MJ, Wagner F, van Lier JJ, et al. Evaluation of T0906487, a CXCR3 antagonist, in a phase 2a psoriasis trial. Inflamm Res 2004;53:S222.
- 109. Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, Wang J, et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 2015 Apr;520(7547):317-321.
- 110. Hollenstein K, Kean J, Bortolato A, Cheng RK, Doré AS, Jazayeri A, et al. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 2013 Jul;499(7459):438-443.