Kawasaki disease (KD) is characterized by an acute febrile, self-limited, generalized multisystem vasculitis that typically affects children aged less than five years.1 The underlying etiology of KD remains unclear. Nonetheless, epidemiological studies suggest that asymptomatic or non-vasculitis infection agents in genetically susceptible individuals could play a role in KD’s pathogenesis.2,3 Patients with KD will develop a variety of clinical manifestations. However, the most important clinical feature is cardiovascular sequels including coronary artery (CA) aneurysm (which occurs in almost 25% of untreated cases), myocardial infarction, arrhythmias, and cardiomyopathy with depression of myocardial contractility.1,4–6 A delay in diagnosis and initiation of appropriate treatment will result in more undesirable outcomes, such as the increased risk of CA aneurysm.7 In addition, injury to myocardial tissue due to excessive inflammation can cause an altered electrical impulse propagation, which manifests through the regional heterogeneity of QT interval duration in myocardial recovery times.8 This inter-lead differences on standard 12-lead electrocardiogram (ECG) is termed QT dispersion.9 Several studies have demonstrated that increase in QT dispersion has an important predictive value for developing potentially lethal arrhythmias and CA aneurysms.10–16 However, the association between QT dispersion and CA involvement among patients with KD has not been widely studied. Therefore, we sought to assess the role of QT dispersion as a practical diagnostic tool for detecting cardiac involvement among patients with KD.
Methods
We conducted a cross-sectional study including all consecutive KD patients who were followed-up at the Pediatric Rheumatology Department (Pediatrics Center of Excellence affiliated to Tehran University of Medical Sciences, Tehran, Iran) between September 2013 and November 2015. A total of 70 patients who met the criteria for KD, based on the American Heart Association guideline, were enrolled in the study.17 The definition for CA involvement was based on the following criteria including abnormal coronary arteries if the internal lumen diameter was > 3 mm in children < 5 years old or > 4 mm in children ≥ 5 years old; if the internal diameter of a segment measured ≥ 1.5 times that of an adjacent segment; or if the coronary lumen was clearly irregular.18 Patients were excluded if they had other causes of cardiac involvement except for CA disease.
We collected data regarding patients’ demographics, clinical manifestations, laboratory, and echocardiographic findings. After diagnosis of KD, all patients received 2 g/kg of intravenous immunoglobulin as a single and high dose of aspirin (80–100 mg).
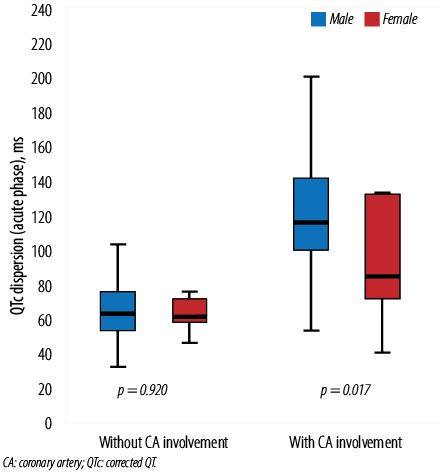
Figure 1: Association between QTc dispersion in the acute phase and CA involvement based on gender. The boxes represent the interquartile range, with the upper and lower edges of the boxes representing the 75th and 25th percentiles, respectively. The central horizontal lines within the boxes represent the median levels for each group.
The ethics committee of Tehran University of Medical Science approved the study and all parents completed an informed consent form before all procedures were performed. The study was conducted following the Declaration of Helsinki and other applicable guidelines, laws, and regulations.19
For each patient, 12-lead ECG at a speed of 25 mm/sec were recorded during the acute phase of the disease. ECG was done on Fokuda (Japan) machine. The QT interval was calculated from the beginning of the QRS complex to the end of the T wave. The end of T wave was defined as the point at which return to baseline, and if the U wave was identified it was excluded from the QT. In cases where the T wave could not be reliably measured, the data was excluded from the study. The QT dispersion was measured as the difference between the maximum and the minimum QT intervals for any of the 12-leads ECG of each patient. The calculation of the corrected QT (QTc) interval was done using the Bazzet’s formula (QTc = QT/square root of the RR interval).20
Continuous variables were examined for a normal distribution using the Shapiro-Wilk test. We used the t-test for independent numeric variables, and for independent nominal variables, Fisher’s exact test or Mann–Whitney’s U test were used, respectively. Pearson’s correlation analysis was used to evaluate the relationship between QTc dispersion and other parameters. We evaluated the suitability of different QTc dispersion for distinguishing between patients with and without CA involvement according to receiver operating characteristic (ROC) curves. The area under the ROC curve (AUC) was calculated with 95% confidence intervals (CI). The cut-off values were defined in line with the optimal sensitivity and specificity. Statistical significance was defined as p < 0.050. Data were analyzed using SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.).
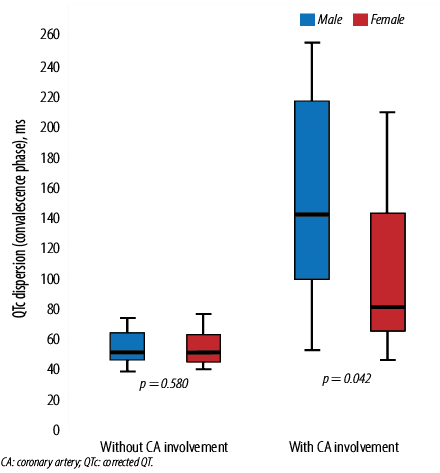
Figure 2: Association between QTc dispersion in the convalescence phase and CA involvement based on gender. The boxes represent the interquartile range, with the upper and lower edges of the boxes representing the 75th and 25th percentiles, respectively. The central horizontal lines within the boxes represent the median levels for each group.
Result
A total of 70 KD patients were identified, including 43 males (61.4%) and 27 females (38.6%). Table 1 describes the baseline demographic and biochemical data of the study cohort. The median age of patients was 21.0 (11.0–48.0) months. The male to female ratio was 1.5:1. We found statistically significant differences between age, gender, and platelet count among patients with and without CA involvement (p < 0.05.0). Median QTc dispersion in patients with CA involvement calculated from 12-lead ECG in the acute phase was 108.0 (89.5–138.5) ms compared to 63.0 (54.0–74.5) ms in the non-CA involvement group (p < 0.001).
Table 1: Clinical characteristics and comparison between patients with and without coronary artery involvement.
|
Age, months |
21.0 (11.0–48.0) |
15.0 (6.5–48.0) |
30.0 (18.0–50.0) |
0.020 |
|
Sex |
|
|
|
0.036 |
|
Male, n (%) |
43 (61.4) |
27 (73.0) |
16 (48.5) |
|
|
Female, n (%) |
27 (38.6) |
10 (27.0) |
17 (51.5) |
|
|
Platelet count, × 103 µL |
560.0 (417.0–722.0) |
653.0 (494.0–1010.0) |
458.0 (376.0–601.0) |
0.001 |
|
CRP |
61.0 (34.0–130.0) |
68.0 (36.0–145.0) |
53.0 (33.0–118.0) |
0.458 |
|
ESR |
80.5 (54.2–102.7) |
76.0 (52.1–104.5) |
81.0 (58.5–103.5) |
0.791 |
|
QTc dispersion, ms |
|
|
|
|
|
Acute phase |
78.5 (60.7–115.0) |
108.0 (89.5–138.5) |
63.0 (54.0–74.5) |
0.001 |
Data presented as interquartile range with 25th and 75th percentiles.
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; QTc: corrected QT.
p-values < 0.050 were considered significant.
*The between-group comparison was made using Mann–Whitney’s U test.
CA abnormality developed in 37 (52.9%) patients, including 15 subjects with small aneurysms (< 5 mm), 16 with medium aneurysms (5–8 mm), and six with giant aneurysms (> 8 mm). A post hoc analysis was implemented to compare between QTc dispersion and various degrees of the severity of CA. The results showed that the mean QTc dispersion was 141.0±62.2, which was significantly higher in patients with medium-sized aneurysms compared to other complications (p < 0.001).
QTc dispersion in both acute and convalescence phases was significantly higher in male subjects compared to females (p = 0.017 and p = 0.042, respectively) [Figure 1 and 2]. Moreover, the Pearson correlation showed that there was a significant positive correlation between QTc dispersion (both acute and convalescence phases) and CA involvement (r = 0.591, p < 0.001 and r = 0.669, p < 0.001, respectively). However, no significant correlation was observed between QTc dispersion and inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein [Table 2 and 3].
Table 2: Correlation between corrected QT dispersion (acute phase) and clinical and laboratory parameters.
|
Age |
0.170 |
0.160 |
|
Gender |
-0.319 |
0.007* |
|
Platelet |
0.160 |
0.186 |
|
CRP |
0.099 |
0.414 |
|
ESR |
-0.087 |
0.472 |
* Statistically significant.
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
Table 3: Correlation between corrected QT dispersion (convalescence phase) and clinical and laboratory parameters.
|
Age |
-0.195 |
0.106 |
|
Gender |
-0.335 |
0.005* |
|
Platelet |
0.176 |
0.144 |
|
CRP |
-0.009 |
0.944 |
|
ESR |
-0.094 |
0.440 |
* Statistically significant.
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
The ROC curve was applied to determine the cut-off values for QTc dispersion in the acute phase with the best sensitivities and specificities for diagnosing CA involvement [Figure 3]. The area under the ROC curve was 0.90 (95% CI: 0.82–0.97), indicating excellent diagnostic accuracy. A cut-off value of 76.5 ms was obtained for QTc dispersion in the acute phase, which gave a sensitivity of 83.8% and a specificity of 80.1%. The AUC for QTc dispersion in the convalescence phase was 0.90 (95% CI: 0.83–0.97), with a cut-off value of 63.5 ms (sensitivity of 83.8% and a specificity of 85.2%) [Figure 4].
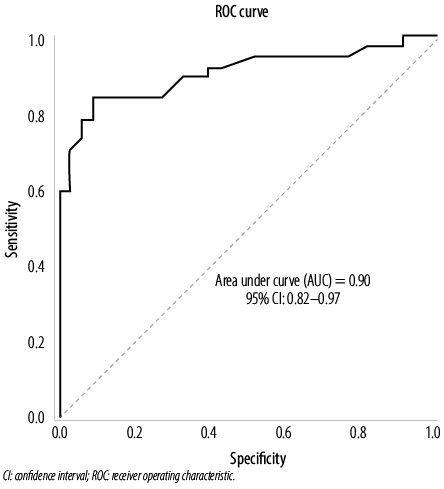
Figure 3: ROC analysis was performed to calculate the AUC, sensitivity, and specificity of the QTc dispersion in the acute phase. The optimal cut-off value for QTc dispersion was 76.5 ms (sensitivity = 83.8% and specificity = 80.1%).
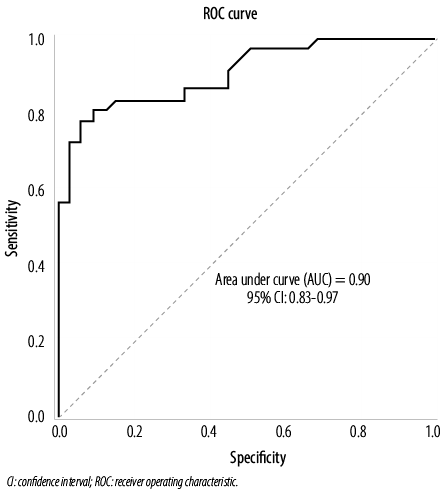
Figure 4: ROC analysis was performed to calculate the AUC, sensitivity, and specificity of the QTc dispersion in the convalescence phase. The optimal cut-off value for QTc dispersion was 63.5 ms (sensitivity = 83.8% and specificity = 85.2%).
Discussion
KD is an acute, self-limited vasculitis and cardiac involvement may occur in untreated cases. The development of CA aneurysm will lead to flow stasis and formation of thrombosis, which could eventually result in myocardial ischemia and infarction. The initial assessment of such complications are evaluated by echocardiography since it is an available and feasible diagnostic tool. Although most patients functional and structural measurements on initial echo may remain unaltered, it cannot detect all types of heart conditions or predict future heart problems.21 Since the recognition of QT dispersion as a prognostic value for heart failure by Barr et al. in 199422, numerous studies have been examined the application of QT dispersion in different medical disciplines.23–26 QT dispersion reflects the heterogeneity of myocardial repolarization that can predict the incidence of ventricular arrhythmia, cardiomyopathy, and sudden cardiac death in both children and adults.15,27,28
The exact underlying mechanism of increased QT dispersion in KD is still unclear. However, two possible explanations for this difference in myocardial recovery times could be suggested. First, the increased QT dispersion may be due to the effect of an abnormal autonomic nervous system described by Kikuchi et al.29 They demonstrated that in KD patients with cardiac involvement, vagal nerve activity was depressed, and therefore resulted in a loss of myocardial stabilization and increased the likelihood of the occurrence of malignant ventricular arrhythmia. Therefore, diminished vagal tone will result in increasing the resistance of peripheral blood flow and subsequently the increase in oxygen demand of the myocardium and may explain the prolongation of the QT interval due to myocardial ischemia. Notably, the current study demonstrated that QT dispersion and QTc dispersion in both the acute and convalescence phases of KD were significantly higher in patients with CA involvement compared to those with no CA involvement. This finding was similar to previous studies, indicating that electrocardiographic depolarization and repolarization would be prolonged in patients with CA involvement.30–32 Moreover, QT dispersion was significantly correlated with CA involvement in our series. However, these findings were in disagreement with previously reported series indicating that no correlation exists between QT interval and CA involvement.11
Secondly, another possible cause of an increased QT dispersion may be related to early pathophysiological changes in the cardiovascular system. Since KD is a multi-systemic vasculitis that affects mostly small and medium-sized arteries, microcirculation disturbances could occur at the coronary microcirculation level even in the absence of endocardial and pericardial alterations. Pathological and clinical evidences suggest that myocardial fibrosis may develop as a result of previous ischemia in the area perfused by the CA.33 Thus, the presence of small areas of myocardial fibrosis may alter the time course of ventricular repolarization, manifested by the existence of dispersion in the effective refractory period.
Previous studies have shown that in patients with primary systemic vasculitides such as systemic lupus erythematosus, Churg-Strauss syndrome, and Behçet’s disease, QT dispersion is associated with cardiovascular involvement.10,25,34–36 Thus, it should be noted that ECG is an essential component of the cardiovascular assessment and measuring QT dispersion could be used as a reliable prognostic test modality for diagnosis of cardiac inflammatory involvement, such as pericarditis, myocarditis, and coronary arteritis, similar to that in KD.37
A previous study reported that the optimal cut-off value of QT dispersion in patients with KD was > 60 ms dispersion with a higher sensitivity in detecting severe coronary aneurysms.31 In contrast, the optimal cut-off value obtained in our study for corrected QT dispersion both in the acute and convalescence phases was 76.5 ms and 63.5 ms, respectively, suggesting that the optimal cut-off value of QTc dispersion with KD is higher than that previously reported. Our results confirmed that the cut-off values differed between the two phases of KD, indicating that different causative mechanisms might account for the prolongation of QTc dispersion in the respective diseases.
The major limitation of our study was its relatively small sample size and the cross-sectional nature of the data. Although our study has achieved a promising result in the application of QT dispersion for early detection of cardiac involvement, whether our findings can be safely extrapolated to all patients with KD should be investigated in a large, prospective, and multicenter study.
Conclusion
Prolonged QT dispersion (corrected or non-corrected) during the acute and convalescence phases in patients with KD is associated with coronary involvement. However, no statistically significant correlation was observed between the repolarization changes and inflammatory markers or disease severity.
Disclosure
The authors declared no conflict of interest. This research was part of a Pediatric Residency thesis (of Dr. L. Hamzehlou) and was approved and financially supported by a grant from Tehran University of Medical Sciences (No: 92-11-165-5006).
Acknowledgements
The authors would like to thank parents who participated in the study.
references
- 1. Petty RE, Laxer RM, Lindsley CB, Wedderburn L. Textbook of pediatric rheumatology. 7th ed. Philadelphia, PA: Elsevier; 2016.
- 2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? IJID 2005 Jul;9(4):185-194.
- 3. Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther 2010 Feb;8(2):197-203.
- 4. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr 1997 Dec;131(6):888-893.
- 5. Lin CY, Lin CC, Hwang B, Chiang BN. Cytokines predict coronary aneurysm formation in Kawasaki disease patients. Eur J Pediatr 1993 Apr;152(4):309-312.
- 6. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al; Pediatric Heart Network Investigators. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation 2007 Jul;116(2):174-179.
- 7. Vijayan AP, Dinesh KB, Nath KR. Coronary artery dilatation in incomplete Kawasaki disease. Indian Pediatr 2009 Jul;46(7):607-609.
- 8. Hung Y, Lin W-H, Lin C-S, Cheng S-M, Tsai T-N, Yang S-P, et al. The prognostic role of QTc interval in acute myocarditis. Acta Cardiol Sin 2016 Mar;32(2):223-230.
- 9. Statters DJ, Malik M, Ward DE, Camm AJ. QT dispersion: problems of methodology and clinical significance. J Cardiovasc Electrophysiol 1994 Aug;5(8):672-685.
- 10. Aytemir K, Ozer N, Aksoyek S, Ozcebe O, Kabakci G, Oto A. Increased QT dispersion in the absence of QT prolongation in patients with Behcet’s disease and ventricular arrhythmias. Int J Cardiol 1998 Dec;67(2):171-175.
- 11. Amoozgar H, Ahmadipour M, Amirhakimi A. QT dispersion and T wave peak-to-end interval dispersion in children with Kawasaki disease. Int Cardiovasc Res J 2013 Sep;7(3):99-103.
- 12. Ghelani SJ, Singh S, Manojkumar R. QT interval dispersion in North Indian children with Kawasaki disease without overt coronary artery abnormalities. Rheumatol Int 2011 Mar;31(3):301-305.
- 13. Parchure N, Batchvarov V, Malik M, Camm AJ, Kaski JC. Increased QT dispersion in patients with Prinzmetal’s variant angina and cardiac arrest. Cardiovasc Res 2001 May;50(2):379-385.
- 14. Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res 2000 Aug;47(2):219-233.
- 15. Motoki N, Akazawa Y, Yamazaki S, Hachiya A, Motoki H, Matsuzaki S, et al. Prognostic significance of QT interval dispersion in the response to intravenous immunoglobulin therapy in Kawasaki disease. Circ J 2017;81(4):537-542.
- 16. Kiani A, Rafieyian S, Roodpeyma S, Sefidgarnia M. The relationship between coronary artery aneurysm and QT interval dispersion in acute phase of Kawasaki disease. Iran J Pediatr 2011 Jun;21(2):220-224.
- 17. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al; Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation
2004 Oct;110(17):2747-2771.
- 18. Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Ministry of Health and Welfare, Tokyo; 1984.
- 19. World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA 2013 Nov;310(20):2191-2194.
- 20. Ahnve S. Correction of the QT interval for heart rate: review of different formulas and the use of Bazett’s formula in myocardial infarction. Am Heart J 1985 Mar;109(3 Pt 1):568-574.
- 21. Hematian MN, Torabi S, MalaKan-Rad E, Sayadpour-Zanjani K, Ziaee V, Lotfi-Tolkaldany M. Noninvasive evaluation of myocardial systolic dysfunction in the early stage of Kawasaki disease: a speckle-tracking echocardiography study. Iran J Pediatr 2015 Jun;25(3):e198.
- 22. Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet 1994 Feb;343(8893):327-329.
- 23. Ueda H, Hayashi T, Tsumura K, Kaitani K, Yoshimaru K, Nakayama Y, et al. QT dispersion and prognosis after coronary stent placement in acute myocardial infarction. Clin Cardiol 2007 May;30(5):229-233.
- 24. Kato T, Kakuta T, Maruyama Y, Hashimoto Y, Yoshimoto N, Numano F. QT dispersion in patients with Takayasu arteritis. Angiology 2000 Sep;51(9):751-756.
- 25. Bornaun HA, Yılmaz N, Kutluk G, Dedeoğlu R, Öztarhan K, Keskindemirci G, Tulunoğlu A, Şap F. Prolonged P-Wave and QT Dispersion in Children with Inflammatory Bowel Disease in Remission. Biomed Res Int. 2017;2017:6960810.
- 26. Tolentino JC, Schmidt SL. Association between depression and cardiovascular disease: A review based on QT dispersion. Eur J Prev Cardiol. 2019 Sep;26(14):1568-1570.
- 27. Spargias KS, Lindsay SJ, Kawar GI, Greenwood DC, Cowan JC, Ball SG, et al. QT dispersion as a predictor of long-term mortality in patients with acute myocardial infarction and clinical evidence of heart failure. Eur Heart J 1999 Aug;20(16):1158-1165.
- 28. Castro-Torres Y, Carmona-Puerta R, Katholi RE. Ventricular repolarization markers for predicting malignant arrhythmias in clinical practice. World J Clin Cases 2015 Aug;3(8):705-720.
- 29. Kikuchi Y, Sato Y, Ichihashi K, Shiraishi H, Momoi MY. Autonomic function in Kawasaki disease with myocardial infarction: usefulness of monitoring heart rate variability. Pediatr Int 2003;45(4):407-409.
- 30. Dahdah NS, Jaeggi E, Fournier A. Electrocardiographic depolarization and repolarization: long-term after Kawasaki disease. Pediatr Cardiol 2002 Sep-Oct;23(5):513-517.
- 31. Osada M, Tanaka Y, Komai T, Maeda Y, Kitano M, Komori S, et al. Coronary arterial involvement and QT dispersion in Kawasaki disease. Am J Cardiol 1999 Aug;84(4):466-468.
- 32. Higham PD, Furniss SS, Campbell RW. QT dispersion and components of the QT interval in ischaemia and infarction. Br Heart J 1995 Jan;73(1):32-36.
- 33. Xie L, Wang R, Huang M, Zhang Y, Shen J, Xiao T. Quantitative evaluation of myocardial fibrosis by cardiac integrated backscatter analysis in Kawasaki disease. Cardiovasc Ultrasound 2016 Jan;14:3.
- 34. Szczeklik W, Sokołowska BM, Mastalerz L, Miszalski-Jamka T, Pacult K, Górka J, et al. QT dispersion in patients with Churg-Strauss syndrome. Kardiol Pol 2011;69(11):1143-1149.
- 35. Cardoso CR, Sales MA, Papi JA, Salles GF. QT-interval parameters are increased in systemic lupus erythematosus patients. Lupus 2005;14(10):846-852.
- 36. Kojuri J, Nazarinia MA, Ghahartars M, Mahmoody Y, Rezaian Gr, Liaghat L. QT dispersion in patients with systemic lupus erythematosus: the impact of disease activity. BMC Cardiovasc Disord 2012 Feb;12:11.
- 37. Farhan HL, Hassan KS, Al-Belushi A, Sallam M, Al-Zakwani I. Diagnostic value of electrocardiographic T wave inversion in lead aVL in diagnosing coronary artery disease in patients with chronic stable angina. Oman Med J 2010 Apr;25(2):124-127.