Ischemic heart disease remains one of the most common leading causes of death worldwide.1 Myocardial infarction (MI) is a common presentation of ischemic heart disease. Permanent loss of cardiomyocytes and scar tissue formation after MI results in irreversible damage to the cardiac tissue and thus impairment of its function. Present medications and procedures have not yet provided the means to regenerate the damaged cardiac muscle and return patients to a pre-heart-attack functional level.2 Thus, cardiac tissue regeneration using stem cells may be an effective therapeutic option.
Isoproterenol (ISO) is a β-adrenoceptor agonist that has been reported to produce severe stress in the myocardium that results in MI if administered in supramaximal doses.3,4 It generates highly cytotoxic reactive oxygen species (ROS) that promote membrane phospholipids peroxidation causing marked impairment of the myocardial membrane and results in infarct-like necrosis of heart muscle.5
Mesenchymal stem cells (MSC) have generated a great promise as potential cells for tissue regeneration. This is primarily owing to their ability to differentiate into mesoderm and non-mesoderm derived tissues and to their unique immune-modulatory effects, thus considered a readily accepted source for allogeneic, ‘off the shelf’ transplantation.6 MSC are readily available from different sources, yet that derived from bone marrow (BM-MSC) and adipose tissue (AT-MSC) are the most heavily investigated.
Promising results have been reported with the use of these two cell sources for cardiac muscle regeneration following acute MI (AMI). Some studies recommend the clinical application potential of AT-MSC, while others conclude that BM-MSC is the most optimal source.7,8 A head-to-head study comparing the two sources while optimizing all study conditions regarding the animal’s species, gender, age, weight, housing conditions, and disease induction, in addition to, the count, phenotype, passage of cells and the route of injection, would give a better comparative assessment for the effectiveness of these two sources in AMI treatment. In this study, MSCs were isolated from bone marrow and adipose tissue to assess their efficacy in cardiac muscle regeneration in AMI rat model.
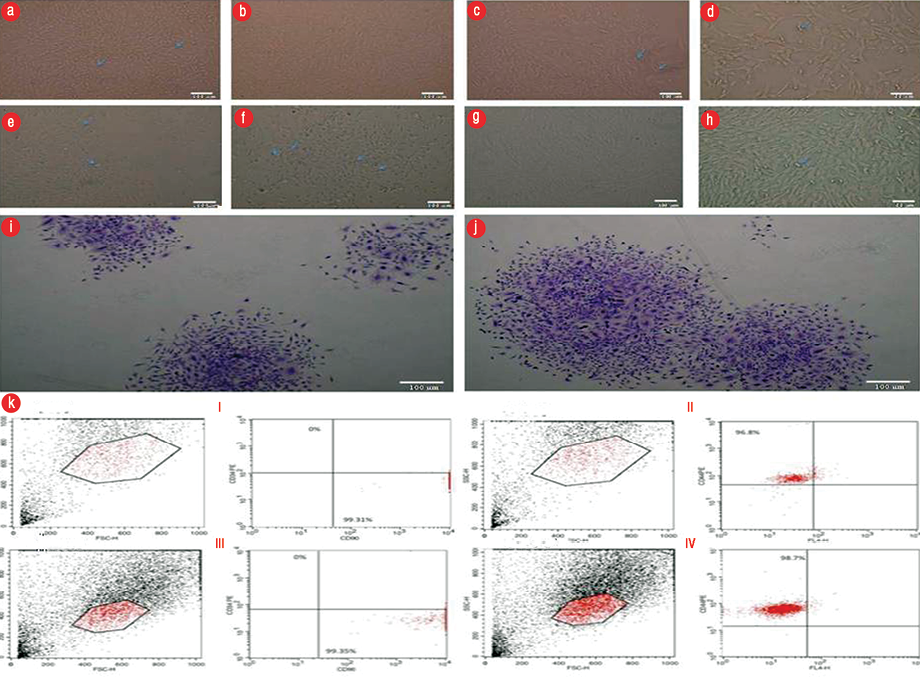
Figure 1: Bone marrow-mesenchymal stem cells (BM-MSC) showed spindle-shaped, fibroblast-like cells (a) primary culture P0, 70.0% confluence, magnification = 100 ×. (b) P1, 70.0% confluence, magnification = 100 ×. (c) P2 fibroblastic cell monolayer, 80.0% confluence, magnification = 100 ×. (d) P2 adipose tissue (AT)-MSC showed spindle-shaped, fibroblast-like cells, magnification = 200 ×. AT-MSC showed spindle-shaped, fibroblast-like cells (e) P0, 20.0% confluence, magnification = 100 ×. (f) P1 50.0% confluence, magnification = 100 ×. (g) Whirlpool-like distribution of P2 monolayer 90.0% confluence, magnification = 100 ×, and (h) magnification = 200 ×. Colony-Forming-Unit assay, P3 Crystal Violet stain (i) BM-MSC showing three colonies (j) AT-MSC showing two colonies. (k) A representative of flow cytometric cell surface markers of P3 cells (I and II,) BM-MSC (III and IV) AT- MSC.
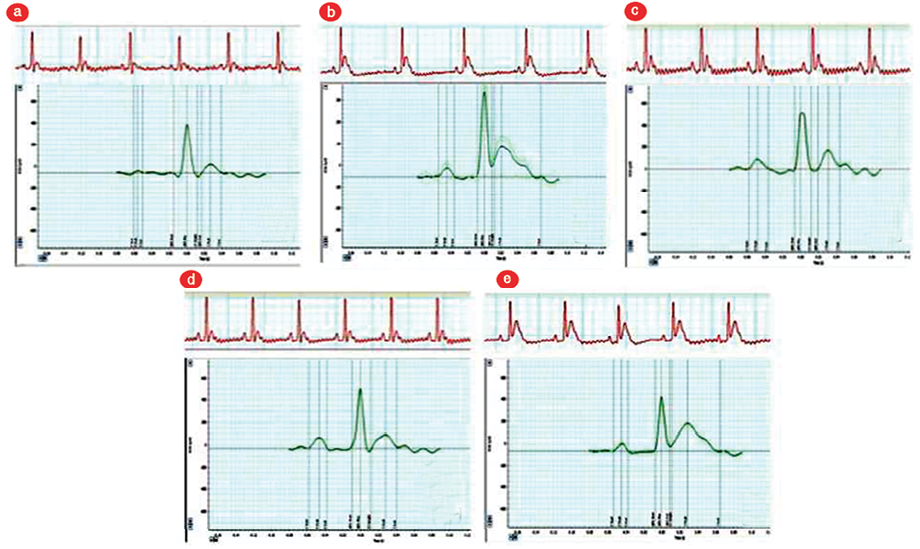
Figure 2: A representative of the electrocardiogram (ECG) view and ECG curve analysis for all groups. (a) control group, (b) acute myocardial infarction (AMI) group,(c) AMI+bone marrow-mesenchymal stem cell group (MSCG), (d) AMI+adiposte tissue (AT)-MSCG, and (e) AMI+cell-free group.
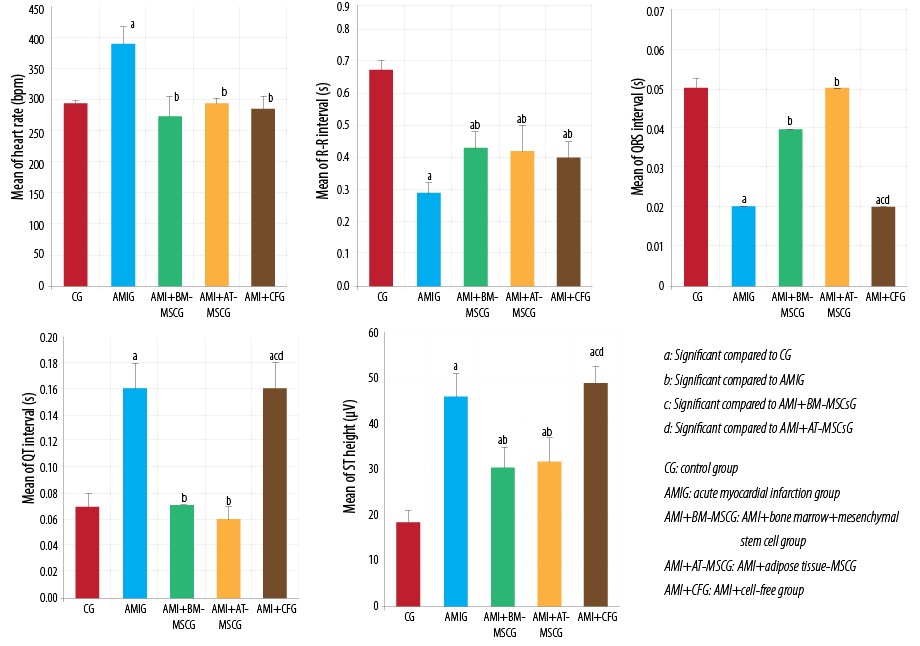
Figure 3: Comparison between studied groups regarding the electrocardiogram parameters using analysis og variance test, Kruskal Wallis test, Tukey post-hoc test, and Dunn’s multiple comparisons test.
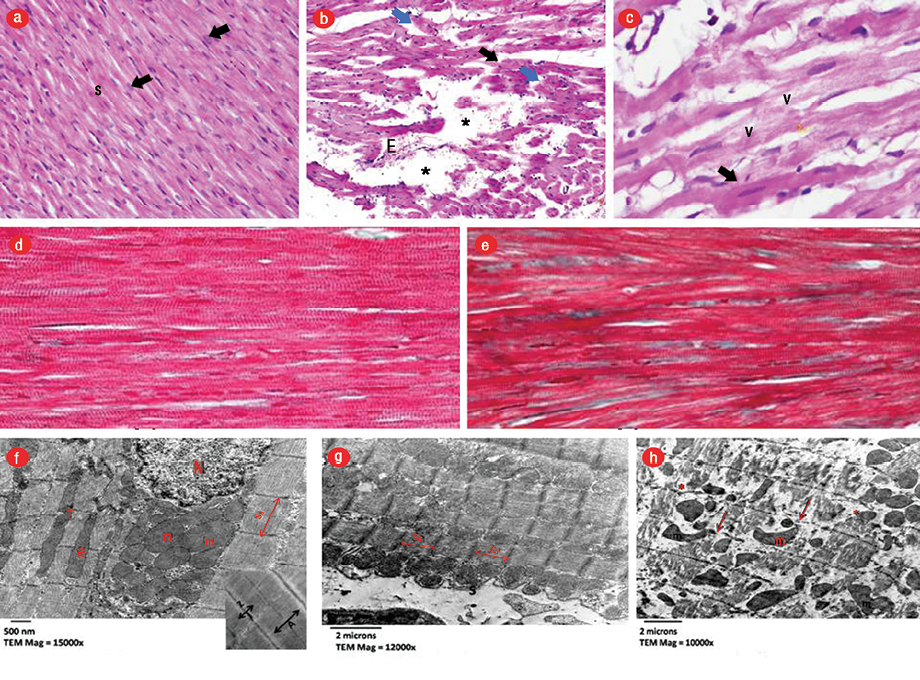
Figure 4: Light micrographs stained by hematoxylin and eosin (H&E). (a) Control group (CG) showing longitudinally arranged fibers with acidophilic cytoplasm and central oval vesicular nuclei (arrows) with slit-like interstitial spaces (S), magnification = 400 ×. (b) AMI group showing wide-spaced thinned (black arrow), discontinued (blue arrow) and fragmented cardiomyocytes with areas of complete fiber loss (asterisks), extravasation of red blood cells, magnification = 400 ×. (c) Focal hypereosinophilic, homogeneous areas with loss of striation (thick arrow) with small dark nuclei. Vacuolated cardiomyocytes (v), magnification = 1000 ×. Masson’s trichrome-stained sections. (d) CG showing normal collagen fibers distribution in between the cardiomyocytes, magnification = 400 ×. (e) AMI group illustrating few collagen fibers in between the cardiomyocytes, magnification = 400 ×. Electron micrographs. (f) CG showing normal architecture of cardiomyocytes with well-ordered myofibrils and sarcomere (sa) and with a central vesicular nucleus (N) and normally arranged mitochondria (m), magnification = 15000 ×. Inset demonstrating alternating dark (A) and light (I) bands and regular Z lines (Z) bisecting I bands, magnification = 20000 ×. (g) AMI group showing markedly shortened and contracted sa, with approximated Z lines, marked scalloping and festooning of the sarcolemma (s), magnification = 12000 ×. (h) Interruption, fragmentation of cardiomyocytes with disturbed myocardial myofibrils pattern with patches of myocytolysis(*), variability of size, shape and abnormal orientation of mitochondria (m). Note, disturbed Z lines (arrows), magnification = 10000 ×.
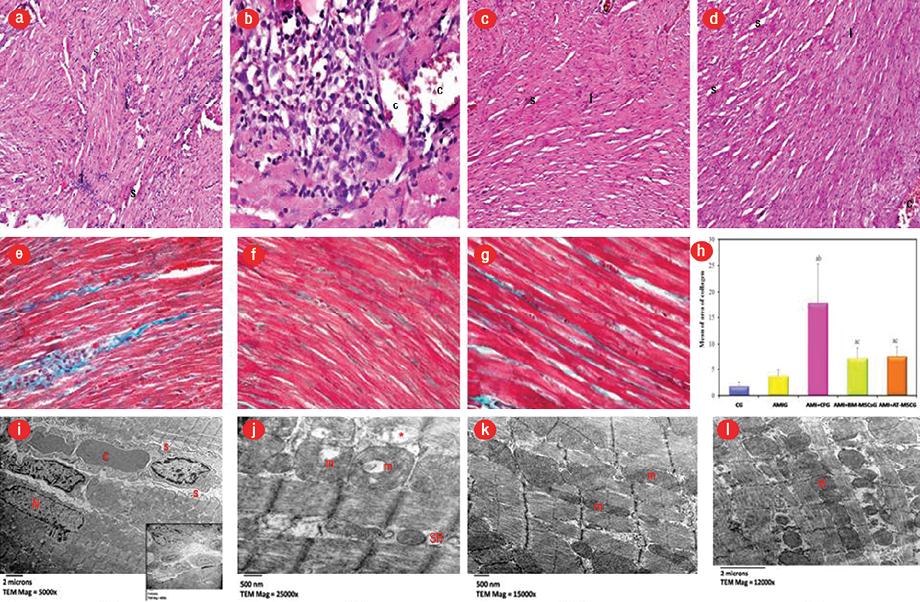
Figure 5: Light micrographs stained by hematoxylin and eosin (H&E). (a and b) acute myocardial infarction+cell-free group (AMI+CFG) showing disorganized widely spaced (S) cardiomyocytes with dense cellular infiltration (I), congested blood vessels (C), magnification =100 × and 400 ×, respectively. (c) AMI+bone marrow-mesenchymal stem cells (BM-MSC) and (d) AMI+adipose tissue (AT)-MSC groups both showed small focal areas of cardiomyocytes disorganization with less spacing (S) in between and mild I. Congestion of blood vessels (C) are still seen but less frequently, magnification = 100 ×. (e) Masson’s trichrome-stained sections showing AMI+CFG marked increase of collagen fibers deposition between the cardiomyocytes and replacing the lost cardiac myocytes, magnification = 400 ×. (f) AMI+BM-MSC and (g) AMI+AT-MSC groups revealed an apparent decrease in collagen fibers between cardiomyocytes, magnification = 400 ×. (h) This was confirmed statistically using the Tukey post-hoc test. (i) Electron micrographs of MI+CF group showing peripheral location of an irregular nucleus (N) with the scalloping of sarcolemma (s) with congested blood capillary (C) in endomysium. Inset demonstrates cellular infiltration, magnification = 5000 ×, and (j) irregular alignment of myofibrils with disrupted myofilaments pattern (*). The mitochondria (m) appeared vacuolated with disoriented and dissolute cristae with dilated sarcotubules (SR), magnification = 2500 ×. However, the myocytes of (k) AMI+BM-MSC and (l) AMI+AT-MSC group revealed more or less normal cardiac myocytes architecture with regularly arranged mitochondria (m) in between, magnification = 1500 × and 1200 ×, respectively.
Methods
MSC were obtained from eight male Sprague-Dawley rats, aged three weeks old. Male rats were used in this study to trace the engraftment of these cells through the demonstration of the SRY gene on the Y chromosome in the hearts of female rats after transplantation. Under sterile conditions, MSC were isolated from bone marrow and adipose tissue, cultured and passaged in vitro. Cells were characterized by their morphology, colony-forming potential, and phenotypes by flow cytometry using fluorescent-labeled monoclonal antibodies for CD44, CD90, and CD34 surface markers. All procedures were conducted in stem cells laboratory in Center of Excellence for Research in Regenerative Medicine and its Application (CERRMA), Faculty of Medicine, Alexandria University.
The study was conducted on 42 female Sprague-Dawley rats, aged six to eight weeks old, weighing 200–220 g. The animals were housed in an animal facility at the Faculty of Medicine, Alexandria University. All experimental procedures were carried out based on the ethical guidelines for care and use of laboratory animals of Alexandria University. (IRB NO: 00007555-FWA NO: 00018699 serial number 020918).
Rats were divided into two groups; the model optimization group (n = 12) and experimental group (n = 30). Rats in the optimization group were further subdivided into the control group (CG; n = 6) and received physiological saline subcutaneously for two consecutive days and the AMI induced group (AMIG; n = 6) received subcutaneous injections of 85 mg/kg of ISO dissolved in 1 mL physiological saline for two consecutive days. Rats of this group were sacrificed 24 hours after last ISO dose. In the experimental group, AMI was induced after the optimization of the model. Twenty-four hours after induction, rats in this group were randomly apportioned into three subgroups: AMI cell-free group (AMI+CFG; n = 10), rats in this group were injected with 1 mL cell-free media (CFM) in the tail vein; bone marrow-derived MSC treated group (AMI+BM-MSC; n = 10); and adipose tissue-derived MSC treated group (AMI+AT-MSC; n = 10) were injected once with 2 × 106 of their respective cell types, intravenously after the induction of AMI. The assessments were done 28 days after injection.
Blood samples were collected from retro-orbital plexus using a microcapillary technique for measurement of creatinine kinase (CK-MB) and lactate dehydrogenase (LDH) enzymes before induction and 24 hours after last ISO dose.
The hearts’ electrical activity was examined using electrocardiogram (ECG) recordings (Powerlab data acquisition system 4/25, AD Instrument, Bella Vista, Australia) before induction, 24 hours after induction, and 28 days after injection of MSCs or CFM.
Ventricular myocardium of all study groups was examined by a light microscope using hematoxylin and eosin (H&E) and Masson’s trichrome stain and by transmission electron microscopy. Morphometric data analysis was done using NIH Fiji© program for estimation of the percentage area of fibrosis.
The genomic DNA extraction was performed using a commercially available kit (Thermo Scientific GeneJET Genomic DNA Purification Kit K0721, K0722). Real-time PCR amplification, data acquisition, and analysis were carried out using the Real-Time detection system Software (Applied Biosystems 7500).
Data were analyzed using SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).9
Results
We observed no morphologic differences between BM-MSC and AT-MSC in culture. Shortly after culturing, we saw fusiform or polygonal cells with multiple small cytoplasmic projections adhering to the tissue culture flask. Cells continued to proliferate displaying a heterogeneous population exhibiting large, flattened, or fibroblast-like shapes forming a monolayer when confluent. Primary culture became 75.0%–85.0% confluent within 8–10 days, with passaging, growth and proliferation rates of cells increase in a way that each passage lasted for 5–6 days until reaching the above mentioned confluence [Figure 1a–h].
The number of colonies displaying five or more cells was scored under the inverted microscope. Colonies that were clearly different from MSC morphology were excluded from the results. The results showed that each well with 100 cells gave 80.0%±3.3 and 78.0%±4.1 of colonies for BM-MSC and AT-MSC, respectively, after 14 days [Figure 1i and j].
BM-MSC expressed mesenchymal CD44 and CD90 in 96.8% and 99.1% of the cultured cells, respectively, and were negative for the CD34 hematopoietic marker. However, AT-MSC demonstrated that 98.7% and 99.4% of the cultured cells expressed the mesenchymal CD44 and CD90 markers, respectively, and were negative for the CD34 hematopoietic marker [Figure 1k].
Forty rats survived the study timeline, showing a mortality rate of 4.8%. Two rats died after induction of MI by ISO, fewer from the injection of stem cells or media.
Induction with ISO lead to a significant increase of serum cardiac enzyme from 242.6±47.8 to 569.3±302.6 for LDH (U/L) and from 395.1±164.4 to 574.3±251.1 for CK-MB (U/L) (p ≤ 0.050).
There was a significant rise in heart rate (as revealed by ECG) in the AMI group compared to the CG (p < 0.010), while it was significantly decreased in AMI+BM-MSC and AMI+AT-MSC groups (p < 0.010) in comparison to AMI group with no significant difference with CG.
AMI group showed significant attenuation of RR and QRS intervals duration, and prolongation of QT interval with significant elevation of the ST segment in comparison to CG.
Treatment with BM-MSC or AT-MSC showed similar results as both treatments significantly restored the RR interval in comparison to the AMI group, although this restoration was not to the normal duration. Similarly, there was a significant increase in the RR duration in AMI+CFG in comparison to AMI. Furthermore, stem cell treated groups showed restoration of QRS interval and QT interval in comparison to the AMI group, with no significant difference compared to the CG. On contrary, there were no changes after injection of CFM.
ST elevation is an important sign of MI. ST segment was significantly elevated in all experimental groups in comparison to the CG. Yet its height was significantly decreased in AMI+BM-MSC and AMI+AT-MSC in comparison to AMI group. On the contrary, there was no significant difference between AMI+CFM group and AMI group and it was significantly higher than BM-MSC and AT-MSC treated groups.
There was no significant difference between AMI+BM-MSC and AMI+AT-MSC in all studied ECG parameters [Figure 2 and 3].
AMI was demonstrated by the loss of normal architecture of cardiomyocytes separated by wide interstitial spacing and cellular infiltration. These findings were also evident in AMI+CFG, and areas of marked increase in collagen deposition were seen. While AMI+BM-MSC and AMI+AT-MSC showed improvement of histological changes compared to AMI+CF [Figures 4 and 5].
Morphometric study showed mild insignificant increase in collagen fibers 24 hours after ISO administration in AMI group compared to the CG (p > 0.050). While 28 days after ISO administration, the percentage of fibrotic areas showed a significant increase in AMI+CFG in comparison with CG and cell treated groups (p < 0.001). On the other hand, in both treated groups the measurement of fibrotic areas was significantly decreased compared to the CFM treated group, but still significantly higher than the CG. There was no significant difference observed between both MSC treated groups regarding the extent of fibrosis.
Real-time PCR revealed that the Y chromosome was expressed in the heart of the female rats injected by male BM-MSC and AT-MSC and not detected in AMI+CFM group [Figure 6].
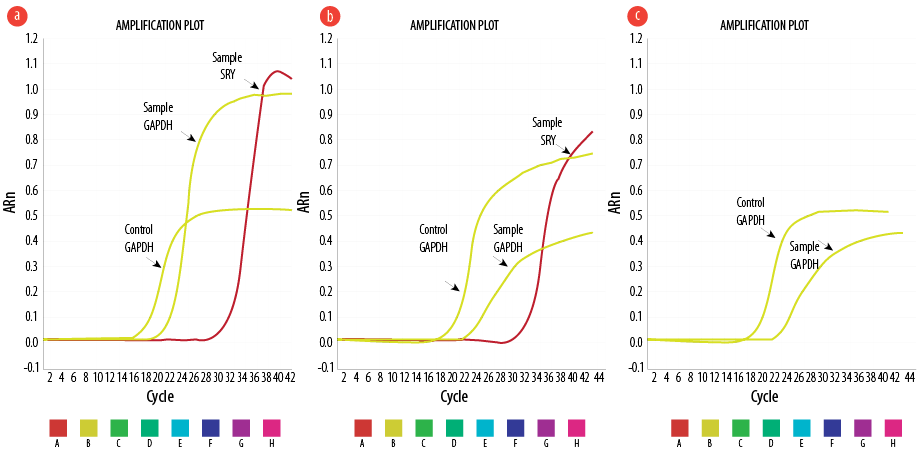
Figure 6: Representative amplification plots for experimental groups illustrating the expression of SRY gene of the Y chromosome in (a) acute myocardial infarction+bone marrow-mesenchymal stem cell (AMI+BM-MSC), (b) AMI+adipose tissue (AT)-MSC, and (c) no expression in AMI+cell-free (CF) group.
Discussion
The successful experimental model of AMI was evaluated by biochemical, ECG changes, and histopathological findings. Biochemical findings in this study were in accordance with the result of Goyal et al,10 who reported that the release of CK-MB and LDH serve as a sign of myocardial injury. This might be due to the damage inflicted upon the sarcolemma by the β-agonist, rendering it leaky or could be secondary events following ISO-induced lipid peroxidation of cardiac membranes, with a consequent increase in enzyme leakage from cardiac myocytes.11
The attenuation of the RR interval in ECG recording reflects myocardial edema and loss of cell membrane integrity, while the QRS interval shows the total duration of ventricular depolarization, its alteration reflects an abnormality of heart function. The QT interval represents the period of electric systole of which is a method of determining the functional integrity of the myocardium.12 QT interval prolongation related to cardiac vagal dysfunction and represents cardiac toxic potential such as an indication of arrhythmias, cardiac dysfunction, and sudden cardiac collapse.13 The ST segment elevation represents the ischemic and non-ischemic zones potential differences and the consequent loss of cell membrane function. ECG pattern alterations induced by isoprenaline detected in this study were previously demonstrated by other investigators.11,14 Raised heart rate induced by ISO may be due to deterioration of mitochondrial energetic and suppression of Ca2+ transport, which causes intracellular Ca2+ overload,15 and also due to deranged sympathetic and parasympathetic input to heart.16
Moreover, histological examination of infarcted myocardium showing coagulative necrosis in the form of disorganization, fragmentation, degeneration and vacuolation of cardiac muscle fibers separated by wide intercellular spaces indicating edema. Similar findings were reported previously.17–19 The wide spacing noticed in our study was in agreement with Patel et al,14 who reported edema induced by capillary leakage that resulted from ROS-induced oxidative damage of ISO. Scalloping of sarcolemma, hypereosinophilia, and the contracted band could be attributed to the intracellular calcium overload leading to activation of protein kinases and impairment of mitochondrial phosphorylating capacity initiating stimulus for myocardial cell death and infarction.11
We injected MSC intravenously the day after induction. According to previous studies, systemic administration of MSC is followed by migration and engraftment of cells into the damaged sites of injury models.20,21 This was in accordance with Cheng et al,22 who reported that systemic infusion of MSCs provides a non-invasive alternative that would be well tolerated by patients with advanced coronary disease or MI. The evidence of MSC homing in the injured heart was proved by the expression of the Y chromosome SRY gene of the injected BM-MSC and AT-MSC isolated from male rats and injected in AMI induced female rats models. The engraftment of these cells could thus lead to myocardial regeneration either by their differentiation or through their paracrine effect that empowers existing and internally recruited progenitors.
Elevated serum cardiac enzymes only persist through acute insult and typically decline a few days after injury. Thus, evidence of regeneration of MI was based solely on two measures: histological and ECG findings. The ECG findings proved that cardiac dysfunction due to ischemic necrosis were significantly improved after 28 days of BM-MSC or AT-MSC injection. There was a restoration to the RR interval duration in BM-MSC, AT-MSC, and CFM treated groups when compared to the AMI group, the restoration of this interval even in cell-free treated group is due to the repair of cell membrane integrity and thus the resolving of resulted edema that occurred after acute injury. On the other hand, QRS, QT intervals, and ST segment changes were only restored after BM-MSC or AT-MSC injections. Alteration of these parameters after ISO denoted cardiac dysfunction, which is due to the loss of cardiomyocytes by necrosis, and thus the restoration of such parameters to reach normal levels denotes the regeneration of cardiac tissue that caused cardiac functions recovery. This is further proved by the absence of such improvement in the CFM treated group as they were indifferent from AMI group and significantly different from control and MSC treated groups. It was noted that there was no significant difference in QT, QRS intervals, and ST segment findings between the BM-MSC and AT-MSC treated groups revealing their equal effectiveness and ability in myocardium regeneration.
Signs of cardiac regeneration were further proved by histological examination, which showed no significant difference between BM-MSC and AT-MSC treated groups. Both demonstrated less cardiomyocytes disorganization with less spacing in between and a mild degree of cardiac muscle fiber degeneration and few cellular infiltrations. We also observed markedly decreased fibrosis, which was confirmed by morphometric analysis.
The signs of cardiac muscle regeneration observed in these two groups may be attributed to the induction of new vessels formation that improves tissue perfusion around the ischemic boundary zone, decrease apoptosis of hypertrophied myocytes in the peri-infarct region, reduced collagen deposition and thus sustain improvement in cardiac function.23 MSCs may contribute to neovascularization in the ischemic myocardium through growth factor-mediated paracrine regulation.24 Also, the differentiation of MSC into cardiomyocytes is another possible mechanism. It was reported by Nagaya et al,25 that intravenous administration of MSCs improves cardiac function after AMI through enhancement of myogenesis in the ischemic myocardium. Moreover, several in vitro studies have shown that BM-MSCs could be programmed to become cardiac myocytes.26
The decreased fibrosis we noticed was in line with a previous study, which reported that MSC have the anti-fibrotic potential for several organs including heart, lung, kidney, and liver.27 It has been postulated that MSCs can reduce cardiac fibroblast proliferation and collagen expression, and they have been able to promote matrix metalloproteinase secretion by cardiac fibroblasts, leading to reduced cardiac ventricular fibrosis. These effects may at least partially be mediated via the release of anti-fibrotic factors such as hepatocyte growth factor.28 This was in accordance with Mias et al,29 who investigated the anti-fibrotic properties of MSC and showed that MSC modulate the phenotype of cardiac fibroblasts and their ability to degrade extracellular matrix.
Our results were in line with Ammar et al,30 who worked on a model of diabetic rats then induced cardiac injury using DOX (doxorubicin hydrochloride) and concluded that BM-MSC and AT-MSC were equally effective in mitigating DOX-induced cardiac damage by promoting angiogenesis, decreasing the infiltration of immune cells and collagen deposition. On the contrary, the left ventricular ejection fraction following MI was improved only after AT-MSC therapy rather than BM-MSC in a study conducted by Rasmussen et al31 who compared hypoxically preconditioned human adipose tissue stem cells and bone marrow stem cells from an elderly patient suffering from coronary atherosclerosis in the treatment of MI using a rat model. Unlike, Karpov et al,32 who evaluated the effect of BM-MSC and AT-MSC transplantation into the peri-infarct area on left ventricular function and myocardial scar size in the rat model of myocardial ischemia-reperfusion. They demonstrated that post-ischemic intramyocardial administration of BM-MSC ameliorated left ventricle function and reduced histological scar size, in contrast to
BM-MSC, AT-MSC transplantation did not affect post-infarction left ventricle remodeling. The different models of cardiac injury used in the previous studies may be behind the controversy of the reported results.
As noted in this study, there was no significant difference in all assessed parameters between the two sources of MSC, and they are both considered effective sources for cardiac regeneration and functional recovery after AMI. Adipose tissue may be more plentiful and easily accessible than bone marrow, but still their regenerative potential is as that of BM-MSCs in AMI model. This may give a free hand to decide the source from which autologous MSCs can be obtained according to the patients’ condition that may favor one source over the other.
Conclusion
Our results demonstrated the effectiveness of MSCs in the treatment of AMI. Furthermore, we were able to demonstrate the equality of the efficiency of MSCs derived from bone marrow and adipose tissue in the AMI model, as both exerted similar beneficial effects in cardiac muscle regeneration and reduction of fibrosis.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Yu LJ, Zhang K-J, Zhu J-Z, Zheng Q, Bao X-Y, Thapa S, et al. Salvianolic acid exerts cardioprotection through promoting angiogenesis in animal models of acute myocardial infarction: preclinical evidence. Oxid Med Cell Longev 2017;2017:8192383.
- 2. Galrinho RD, Manole CG, Vinereanu D. Telocytes - a hope for cardiac repair after myocardial infarction. Maedica (Buchar) 2016 Dec;11(4):325-329.
- 3. Afroz R, Tanvir EM, Karim N, Hossain MS, Alam N, Gan SH, et al. Sundarban honey confers protection against isoproterenol-induced myocardial infarction in wistar rats. Biomed Res Int 2016;2016:6437641.
- 4. Lobo Filho HG, Ferreira NL, Sousa RB, Carvalho ER, Lobo PL, Lobo Filho JG. Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Cir Cardiovasc 2011 Jul-Sep;26(3):469-476.
- 5. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, et al. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J Pineal Res 2010 Apr;48(3):251-262.
- 6. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 2014 Mar;32(3):252-260.
- 7. Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther 2015 Apr;6:55.
- 8. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012 Sep;21(14):2724-2752.
- 9. Kotz SB, Read CB, Vidakovic B. Encyclopedia of statistical sciences. 2nd ed. Hoboken, N.J.: Wiley-Interscience; 2006.
- 10. Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S, et al. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci 2015 Nov;16(11):27457-27469.
- 11. Baky NA, Al-Rasheed NM, Al-Rasheed NM, Zaghloul IY, Radwan MA. Alpha-lipoic acid and amlodipine ameliorate myocardial infarction induced by isoproterenol in rats. Int J Acad Res 2009;1:68-77.
- 12. Thippeswamy BS, Tubachi S, Kalyani GA, Netra MK, Patil U, Desai S, et al. Cardioprotective effect of cucumis trigonus roxb on isoproterenol-induced myocardial infarction in rat. Am J Pharmacol Toxicol 2009;4(2):29-37.
- 13. Mazzoleni A, Curtin ME, Wolff R, Reiner L, Somes G. On the relationship between heart weights, fibrosis, and QRS duration. J Electrocardiol 1975 Jul;8(3):233-236.
- 14. Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol 2010 Oct;644(1-3):160-168.
- 15. Goyal S, Arora S, Mittal R, Joshi S, Nag TC, Ray R, et al. Myocardial salvaging effect of telmisartan in experimental model of myocardial infarction. Eur J Pharmacol 2009 Oct;619(1-3):75-84.
- 16. Xenophontos XP, Watson PA, Chua BH, Haneda T, Morgan HE. Increased cyclic AMP content accelerates protein synthesis in rat heart. Circ Res 1989 Sep;65(3):647-656.
- 17. Al-Rasheed NM, Al-Oteibi MM, Al-Manee RZ, Al-Shareef SA, Al-Rasheed NM, Hasan IH, et al. Simvastatin prevents isoproterenol-induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Des Devel Ther 2015 Jun;9:3217-3229.
- 18. Prasad EM, Mopuri R, Islam MS, Kodidhela LD. Cardioprotective effect of Vitex negundo on isoproterenol-induced myocardial necrosis in wistar rats: A dual approach study. Biomed Pharmacother 2017 Jan;85:601-610.
- 19. Nour MS, Sarhan NR, Mazroa SA, Gawish SA. Histological and immunohistochemical study of cardiac telocytes in a rat model of isoproterenol-induced myocardial infarction with a reference to the effect of grape seed extract. Acta Histochem 2017 Sep;119(7):747-758.
- 20. Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 2003 Dec;5(12):1028-1038.
- 21. Zickri MB, Embaby A, Metwally HG. Experimental study on the effect of intravenous stem cell therapy on intestinal ischemia reperfusion induced myocardial injury. Int J Stem Cells 2013 Nov;6(2):121-128.
- 22. Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 2008 Mar;16(3):571-579.
- 23. Bai W-W, Xing Y-F, Wang B, Lu X-T, Wang Y-B, Sun Y-Y, et al. Tongxinluo Improves Cardiac Function and Ameliorates Ventricular Remodeling in Mice Model of Myocardial Infarction through Enhancing Angiogenesis. Evid Based Complement Alternat Med 2013;2013:813247.
- 24. Santos Nascimento D, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther 2014 Jan;5(1):5.
- 25. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 2004 Dec;287(6):H2670-H2676.
- 26. Helal O, El-Mansy A, El-khair WA. Role of stem cells in regeneration of myocardium in experimentally induced myocardial infarction. Egypt J Histol 2010;33(1):8-16.
- 27. Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int 2014;2014:340257.
- 28. Van Linthout S, Stamm Ch, Schultheiss HP, Tschöpe C. Mesenchymal stem cells and inflammatory cardiomyopathy: cardiac homing and beyond. Cardiol Res Pract 2011 Mar;2011:757154.
- 29. Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas M-H, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009 Nov;27(11):2734-2743.
- 30. Ammar HI, Sequiera GL, Nashed MB, Ammar RI, Gabr HM, Elsayed HE, et al. Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res Ther 2015 Aug;6:148.
- 31. Rasmussen JG, Frøbert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant 2014 Feb;23(2):195-206.
- 32. Karpov AA, Uspenskaya YK, Minasian SM, Puzanov MV, Dmitrieva RI, Bilibina AA, et al. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int J Exp Pathol 2013 Jun;94(3):169-177.