Thrombocytosis in children and adults is defined as an elevated platelet count ≥ 450 × 109/L.1–3 A normal platelet count ranges between 150–450 × 109/L which is generally accepted for healthy neonates, infants, children, and adolescents.1,2 The causes of elevated platelet count in children and adults can be primary or secondary in origin.2–4 Primary thrombocytosis can be due to bone marrow disease, for example, myeloproliferative neoplasm (essential thrombocythemia, polycythemia vera, and others) as shown in Table 1. Secondary (or reactive) thrombocytosis refers to elevated platelet count in the absence of the primary causes.1–3 The latter is secondary to various medical or surgical conditions.3–5 The most common reactive causes of thrombocytosis are infection, inflammation, iron deficiency, tissue damage, hemolysis, malignancy, hyposplenism, and other conditions of acute phase response [Table 1].2
Thrombocytosis secondary to a reactive cause is usually easily reversible.3 Classification of thrombocytosis includes mild (450–700 × 109/L), moderate (700–900 × 109/L), and severe thrombocytosis (> 900 × 109/L).1,6 Extreme thrombocytosis is defined as a platelet count ≥ 1000 × 109/L.1,6 Extreme thrombocytosis is an uncommon finding, especially among pediatric patients. Therefore, different causes of thrombocytosis in childhood were investigated, after considering the possible differential diagnoses in this age group through an extensive literature search.
Table 1: Causes of thrombocytosis in adults and children.
|
Essential thrombocythemia |
Infection |
|
Polycythemia vera |
Inflammation |
|
Primary myelofibrosis |
Tissue damage |
|
Myelodysplasia with del (5q) |
Hyposplenism |
|
Refractory anemia with ring sideroblasts associated with marked thrombocytosis* |
Post-operative |
|
Chronic myelomonocytic leukemia* |
Hemorrhage |
|
Atypical chronic myeloid leukemia/ childhood* |
Iron deficiency |
|
Myelodysplastic/myeloproliferative neoplasms, unclassifiable* |
Hemolysis |
|
Familial thrombocytosis |
Drug therapy (e.g., corticosteroids, adrenaline) |
| |
Cytokine administration (e.g., thrombopoietin) |
| |
Rebound following myelosuppressive chemotherapy/splenectomy |
*Rarely seen in children.
Case report
A five-year-old boy presented with recurrent episodes of spontaneous epistaxis. Each episode lasted for a few seconds with minimal bleeding. However, there was no other bleeding manifestation. He had no history of trauma (such as fall or nose picking) and no history of fever. His vital signs were normal. He had no family history of hematological disorder. His parents and siblings had normal platelet counts. His physical examination revealed palpable shotty cervical lymph nodes bilaterally, but no hepatosplenomegaly. Laboratory results at presentation showed hemoglobin of 11.4 g/dL, total leukocyte count of 10 × 103/µL, and mild peripheral eosinophilia (0.92 × 109/L) with a platelet count of 2333 × 109/L [Table 2]. The peripheral blood smear confirmed thrombocytosis showing packed platelets with significant platelet anisocytosis. No significant red cell fragments and cell debris were seen. His bone marrow aspirate showed hypercellular marrow with megakaryocytic proliferation and mild dysmegakaryopoiesis as shown by hypolobated nucleus of some megakaryocytes and some megakaryocytes were seen in clusters. No other nuclei abnormalities were detected.7 Increased eosinophils and their precursors were seen but no increase in blasts cells [Figure 1 and 2]. Myeloid and erythroid were normal in cellularity. Unfortunately, trephine biopsy samples were suboptimal for interpretation, hence, reticulin stain is unavailable. The parents did not agree to repeat marrow examination. The Janus kinase 2 (JAK2) V617F mutation and BCR/ABL fusion gene were not detected. Cytogenetic analysis was reported normal.
Table 2: Laboratory investigation results of the patient.
|
Hematological and coagulation test |
|
Serum ferritin |
42.04 µg/L (normal) |
14.45–87.75 µg/L |
|
Prothrombin time |
14.2 sec (normal) |
12.6–15.7 sec |
|
Activated partial thromboplastin time |
40.1 sec (normal) |
30.0–45.8 sec |
|
Fibrinogen |
2.2 g/L (normal) |
2.2–4.4 g/L |
|
Inflammatory and infection work-up |
|
C-reactive protein |
< 10 mg/L (normal) |
< 10 mg/L |
|
Uric acid |
239 µm/L (normal) |
120–320 µm/L |
|
Stool for ova and cyst |
Negative |
- |
|
Stool for occult blood |
Negative |
- |
|
Blood for culture and sensitivity |
Negative |
- |
|
Lactate dehydrogenase (LDH) |
769 U/L (increased) |
< 480 U/L |
|
Liver function tests |
|
|
|
Albumin |
42 g/L (normal) |
38–42 g/L |
|
Aspartate transaminase |
31 U/L (normal) |
5–35 U/L |
|
Alanine transaminase |
13 U/L (normal) |
13–45 U/L |
|
Renal function tests |
|
|
|
Sodium |
135 mmol/L (normal) |
135–145 mmol/L |
|
Potassium |
3.6 mmol/L (normal) |
3.5–5.0 mmol/L |
|
Urea |
4.3 mmol/L (normal) |
1.7–8.3 mmol/L |
Other investigations for secondary eosinophilia, including stool for ova and cysts as well as connective tissue disease screening were negative. The patient was started on aspirin by the pediatrician due to the very high platelet count. He was closely monitored to minimize the potential risk of bleeding and thrombosis. The patient is currently under follow-up at the pediatric outpatient clinic, and his serial platelet counts showed reducing trend. His latest platelet count, seven months after his initial presentation, was 463 × 109/L. The patient is well, there have been no new findings, and aspirin treatment has been stopped. After reviewing all available investigations, the cause of extreme thrombocytosis in this patient is most likely reactive. However, the patient should be reevaluated to rule out thrombocytosis since his platelet count is still slightly above normal range. We would consider conducting other molecular tests to detect gene mutations such as calreticulin (CALR), myeloproliferative leukemia, FIPL1, and JAK2 exon 12 if the platelet count was persistently high during follow-up, especially if eosinophilia was still present and his lactate dehydrogenase levels also increased.
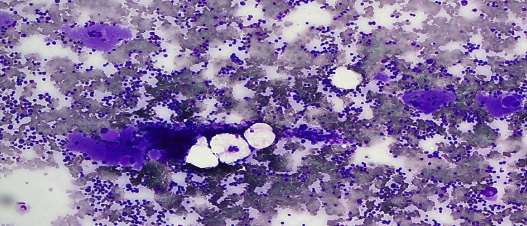
Figure 1: Bone marrow aspirate stained with May-GrÜnwald Giemsa stain showing marrow hypercellularity with increased megakaryocytes. Magnification = 10 ×.
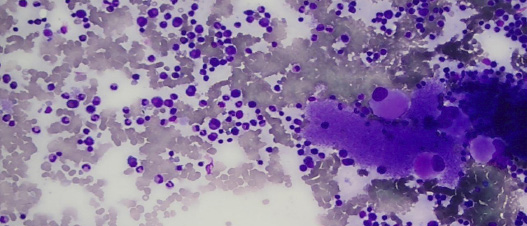
Figure 2: Bone marrow aspirate showing plentiful megakaryocytes and increased eosinophils and their precursors. Magnification = 40 ×.
Discussion
Thrombocytosis is a common finding in children, which is often transient and occurs secondary to various underlying medical conditions.8–13 It has been reported to occur in hospitalized children; most had mild thrombocytosis, but others transiently reached levels over 900 × 109/L.2,8–10 Furthermore, thrombocytosis can also be detected in children during routine blood checkup, usually due to a recent self-limited infection or inflammation.10–12 After a few weeks, a repeat full blood count will commonly show normalization or at least a reduced platelet count.10,11 In most children with thrombocytosis, the clinical symptoms of underlying systemic disease can be clearly seen.1,2,12 However, there are few cases, where differentiating between primary and reactive causes of thrombocytosis can be very difficult as seen in our case.1–3
The most interesting feature for this case was extreme thrombocytosis. Different causes of thrombocytosis in childhood should be ruled out. With proper history taking, thorough physical examination, and further investigation, it would be possible to narrow down to the possible differential diagnosis [Figure 3]. Investigations for this patient did not reveal any evidence of a primary hematological disorder. Lactate dehydrogenase was increased in this patient most probably due to reactive causes.14 The patient remained well, and the latest platelet count showed reduction to a near normal level. However, he remains under regular follow-up.
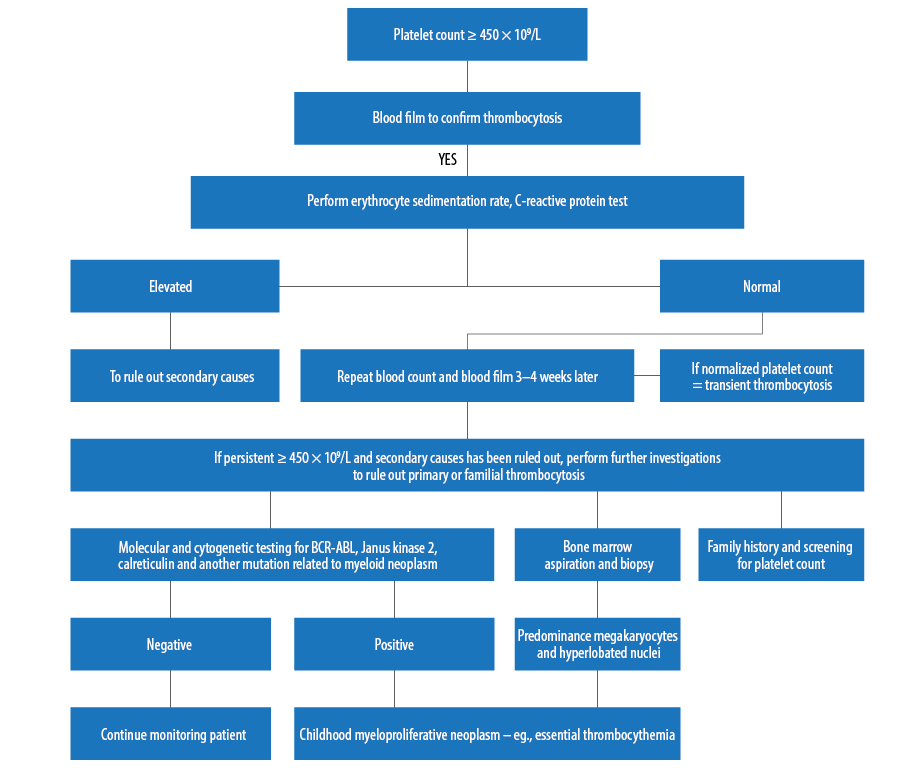
Figure 3: Suggested approaches of laboratory investigations for childhood thrombocytosis.
Primary thrombocytosis is rare in children.8 Essential thrombocythemia is over 60-times more common in adults than children.1,8 Primary thrombocytosis is caused by monoclonal or polyclonal abnormalities of hematopoietic cells or by abnormalities in the biology of thrombopoietin (TPO).2 TPO is the hormone responsible for platelet production in humans and is mainly produced by the liver and kidneys.11,14 Secondary thrombocytosis results from increased thrombopoiesis and caused by stimulated megakaryopoiesis from various underlying hematological or non-hematological disorders.2,10
Another differential diagnosis to be ruled out is familial thrombocytosis (FT), a rare autosomal dominant hereditary form of thrombocythemia. To date, many families with FT have been reported.15 FT is caused by a mutation in the promoter of the TPO gene, which encodes TPO.16 Family studies should be performed in unexplained persistent childhood thrombocytosis as was done in this case.16 The cause of epistaxis in this child could be due to acquired platelet disorder of eosinophilia. However, he did not experience any other episodes of bleeding subsequently.
It is difficult to use the cut-off of platelet count as a predictor of whether the thrombocytosis is primary or secondary.11 Extreme thrombocytosis in children is commonly a benign and reactive phenomenon.9 If a primary cause has been excluded, the possibility of reactive thrombocytosis should be considered, and initiating appropriate treatment will usually result in normalization of the platelet count.10
Conclusion
This case report illustrates the approach to the management of children with extreme thrombocytosis. It is reasonable to give time for follow-up for a patient with extreme thrombocytosis if the patient is clinically well and no other significant clinical findings such as hepatomegaly and other organ enlargement were reported. The case also demonstrates the challenges to diagnose the case of extreme thrombocytosis in a child. The causes in children are different from those encountered in adults where underlying primary hematological disorders are more common.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Dame C, Sutor AH. Primary and secondary thrombocytosis in childhood. Br J Haematol 2005 Apr;129(2):165-177.
- 2. Harrison CN, Bareford D, Butt N, Campbell P, Conneally E, Drummond M, et al; British Committee for Standards in Haematology. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 2010 May;149(3):352-375.
- 3. Hamza H, Umayr A. Extreme thrombocytosis: A unique constellation of reactive thrombocytosis, iron deficiency anemia and atypical celiac disease. American Journal of Medical Case Reports. 2015;3(6):184-186.
- 4. Aladily TN, Mohammad RS, Al-Khader A, Awidi AS. Essential thrombocythemia in a two-year-old child, responsive to hydroxyurea but not aspirin. Oman Med J 2017 May;32(3):243-246.
- 5. Deaconu A, Bica C, Bulucea D. Reactive thrombocytosis in pediatric Pathology. Medicina (B Aires) 2014;21:51-54.
- 6. Sutor AH. Thrombocytosis. In: Lilleyman JS, Hann IM, Blanchette VS, editors. Pediatric hematology. 2nd ed. Churchill Livingstone, London;1999. p. 455-464.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. IARC: Lyon; 2017.
- 8. Chandna A, Sharma J, Sangwaiya A, Samal S, Sankala M, Garg S. Iron deficiency anemia with thrombocytosis: a diagnostic challenge. International J. of Healthcare and Biomedical Research. 2014;3:43-46.
- 9. Kucine N, Chastain KM, Mahler MB, Bussel JB. Primary thrombocytosis in children. Haematologica 2014 Apr;99(4):620-628.
- 10. Chan KW, Kaikov Y, Wadsworth LD. Thrombocytosis in childhood: a survey of 94 patients. Pediatrics 1989 Dec;84(6):1064-1067.
- 11. Mantadakis E, Tsalkidis A, Chatzimichael A. Thrombocytosis in childhood. Indian Pediatr 2008 Aug;45(8):669-677.
- 12. de Lama Caro-Patón G, García-Salido A, Iglesias-Bouzas MI, Guillén M, Cañedo-Villaroya E, Martínez-Romera I, et al. [Extreme reactive thrombocytosis in a healthy 6 year-old child]. An Pediatr (Barc) 2014 Nov;81(5):318-321.
- 13. Sudhakar MK, Senthil N, Mohini S, Hissham. A case of reactive thrombocytosis. Sri Ramachandra Journal of Medicine 2007;1:62-63.
- 14. Farah S, Ahmad S, Soriyash F, Farzaneh S. Elevated serum lactate dehydrogenase values in children with multiorgan involvements and severe febrile illness. Iranian Journal of Paediatric Society 2007;1:31-35.
- 15. Fujiwara T, Harigae H, Kameoka J, Yokoyama H, Takahashi S, Tomiya Y, et al. A case of familial thrombocytosis: possible role of altered thrombopoietin production. Am J Hematol 2004 Aug;76(4):395-397.
- 16. Bain BJ. Blood cells: a practical guide, 5th Edition. Wiley Blackwell 2015.