Catheter-related bladder discomfort (CRBD) is one of the serious problems in the postoperative period. It presents as urinary urgency and frequency in the recovery room. The incidence of CRBD has been reported as high as 47% in some studies.1,2 Many studies are applying various methods to reduce the symptoms of CRBD following catheter insertion.3,4
CRBD is due to muscarinic receptor-mediated involuntary contractions of the bladder smooth muscle. Therefore, agents with antimuscarinic properties, such as oxybutynin, tolterodine, and gabapentin are used to relieve it. Additionally, intraoperative administration of tramadol and ketamine has been reported to reduce the incidence and severity of CRBD following surgery.5,6 We have already examined and found promising results regarding the efficacy of ketamine administration on the reduction of CRBD, but the increased incidence of adverse effects attributed to ketamine (such as psychotic episodes) that may be seen in the recovery room is unacceptable. We sought to determine whether coadministration of haloperidol can reduce the adverse effects attributed to ketamine while keeping its desired effects on CRBD.
Methods
We conducted a prospective, randomized, double-blinded trial. The study was registered at the IRCT website (IRCT20171012036730N2). One hundred and twenty male patients undergoing elective lumbar spinal stenosis surgery assessed for eligibility and were included in the study if they had an American Society of Anesthesiologists (ASA) status I or II and were aged 18 to 70 years old. The CONSORT’s flowchart is depicted in Figure 1. The study was approved by the ethics committee of the university research deputy. Written informed consent was obtained from all patients enrolled in the study. Patients were divided into three groups using a table of random numbers created by computer software.

Figure 1: Consort’s flow diagram of the study.
Patients were excluded if they displayed any of the following criteria: failure to extubate, history of urological surgery, damage or distracting pain other than site of surgery, history of chronic pain, history of opium or other substance use, history of urinary system obstruction, history of known urinary tract infection or urinary stones, history of severe heart or liver disease or long QT interval in electrocardiogram (ECG), history of overactive bladder (urinating more than three times at night or more than eight times a day), morbid obesity (body mass index (BMI) > 40), history of dementia or any disorder that results in inappropriate orientation and inability to communicate verbally, patients with end-stage renal disease (urinary volume less than 500cc in 24 hours), history of any apparent bladder dysfunction due to lumbar spinal cord stenosis, history of sensitivity to phenylephrine, ketamine or pethidine, or a history of monoamine oxidase inhibitor (MAOI) consumption in the last two weeks.
The ketamine-haloperidol (KH) group included 39 patients who received KH just before urinary catheterization. Another 40 patients received a combination of haloperidol and pethidine and were included in the pethidine-haloperidol (PH) group. The control (C) group consisted of 40 patients receiving saline as a placebo. Anesthesia was performed for all patients using the same method: injecting fentanyl 1.0 µg/kg, midazolam 0.05 mg/kg, thiopental sodium 5.0 mg/kg, atracurium 1.0 mg/kg, and lidocaine 1.0 mg/kg. Anesthesia was maintained using isoflurane in an air/oxygen mixture and a bolus injection of fentanyl 1.0 µg/kg hourly.
Immediately after induction of anesthesia, 5 mL normal saline was intravenously (IV) administered for patients in C group. Patients in the KH group were given 0.04 mg/kg haloperidol plus 0.5 mg/kg ketamine IV in a 5 mL syringe, and patients in the PH group were given 0.04 mg/kg haloperidol plus 0.5 mg/kg pethidine IV in a 5 mL syringe. Patients and those who injected the drugs and performed assessments were blinded to the drug administered (an anesthesia technician had prepared all drugs). For all patients, urinary catheterization was performed in sterile conditions using a 16 Fr. Foley’s catheter 120 seconds after the intervention to induce preemptive analgesia before catheterization. A water-based gel was used to lubricate the catheter. Urinary catheters were left for free drainage up to 24 hours after the operation and then removed. All patients were positioned to the prone condition, ensuring that the pathway of the catheter for each patient was intact and the free flow of urine was not obstructed.
Intraoperative standard monitoring including a five-lead electrocardiogram, noninvasive blood pressure, pulse oximeter, core body temperature, and end-tidal carbon dioxide were performed. Patient’s respiratory rate (RR) was also monitored. After completion of the operation, the neuromuscular blockade was reversed using a combination of neostigmine 40.0 µg/kg and atropine 20.0 µg/kg. Patients were transferred to the recovery room after fulfilling extubation criteria.
The occurrence of CRBD as an unpleasant sensation in the suprapubic area and the sense of urgency were recorded on arrival in the recovery room in each patient by a person who was blind to injected drugs as the primary outcome of the study. Using a four-stage scale, CRBD severity was recorded at one, six, and 24 hours with a score of 0 indicating no complaint of pain at all, 1 = mild discomfort, 2 = moderate spontaneous pain, and 3 = severe pain, which causes behavioral response such as flailing limbs, a strong verbal response, and attempts to pull out the catheter.
Additionally, the level of sedation and untoward events such as hallucination, postoperative nausea and vomiting (PONV), respiratory depression (RR < 8 or oxygen saturation < 90%), diplopia, hypotension (systolic blood pressure < 90 and diastolic blood pressure < 60), headache, and skin rash were also recorded. The Ramsay sedation scale was used to measure the level of sedation and was defined as follows: 1 = anxious, agitated, and restless; 2 = cooperative, oriented, and calm; 3 = sleepy and responding to commands; 4 = rapid and strong response to light glabellar tap or a loud noise; 5 = slow response to light glabellar tap or a loud noise; and 6 = no response to light glabellar tap or a loud noise. Patients with a score higher than 4 were considered sedated. The level of pain in the surgical site in each patient was recorded using the visual analog scale (VAS) after surgery, upon arrival in the recovery room, and one, six, and hours after. Apotel (Uni-Pharma Kleon Tsetis Pharmaceutical Laboratories, S.A, Czech) was administered (20.0 mg/kg up to maximum 1.0 g) for postoperative patients if their VAS score was greater than 4. If Apotel could not reduce their VAS score to less than 4, then morphine 0.05 mg/kg IV was administered as the second-line painkiller. The duration of surgery was also recorded for each patient.
All statistical analysis was done using SPSS Statistics (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc). The primary outcome was the incidence of CRBD in the recovery room.
Baseline data were presented as mean±standard deviation for quantitative variables. An independent sample t-test and repeated measure analysis of variance were used to compare the main outcome measures between the study groups and a p-value < 0.050 was considered significant.
Results
A total of 119 male patients who were candidates for lumbar spinal stenosis surgery were eligible for inclusion in the study. They were aged 18 to 70 years old [Table 1].
Table 1: Demographic data of patients and mean surgery durations.
|
Age, years |
43.3 ± 13.8 |
45.8 ± 15.5 |
45.1 ± 13.7 |
0.718 |
|
ASA grade |
1.10 ± 0.3 |
1.1 ± 0.3 |
1.17 ± 0.38 |
0.526 |
|
Weight, kg |
71.4 ± 13.1 |
74.1 ± 10.0 |
75.8 ± 12.0 |
0.265 |
|
Height, cm |
174.6 ± 10.8 |
174.2 ± 11.8 |
170.9 ± 11.3 |
0.274 |
ASA: American Society of Anesthesiologists; KH: ketamine-haloperidol; PH: pethidine-haloperidol; C: control.
The incidence of CRBD was 55% in the C group while it was 17.9% and 52.5% in KH and PH groups, respectively. The severity of CRBD was significantly lower in the KH group one and six hours after surgery (p < 0.007). The mean severity score of CRBD was lower at 24 hours in the KH group, but this difference was not statistically significant [Figure 2].The severity of surgical pain was compared among the three groups with no significant difference observed [Figure 3].
Patients sedation level upon arrival in the recovery room, and one, six, and 24 hours after surgery were compared with no significant difference among groups [Figure 4].
Also, PONV, hallucination, diplopia, headache, skin rash, respiratory dysfunction, and hypotension were evaluated among groups. There were three cases out of 39 patients within the KH group that developed PONV, four cases out of 40 patients within the PH group, and two cases out of 40 patients in the C group. We found no statistically significant difference between the groups. None of the cases developed hypotension, hallucination, diplopia, headache, skin rash, or respiratory dysfunction in recovery.
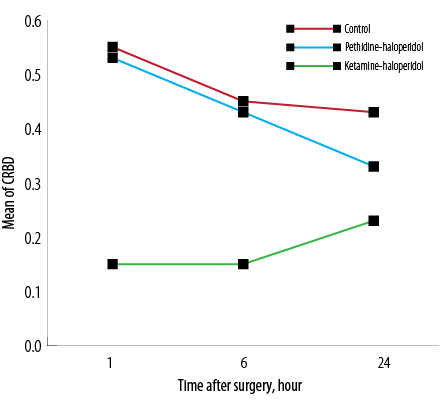
Figure 2: The mean catheter-related bladder discomfort (CRBD) severity score in the three groups at one, six, and 24 hours post-surgery.
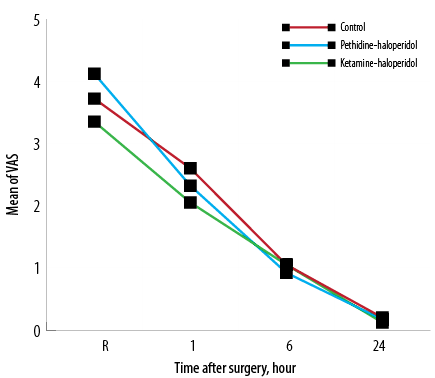
Figure 3: Mean pain intensity using the visual analog scale (VAS) at the site of operation upon arrival in the recovery room (R) and one, six, and 24 hours after surgery.
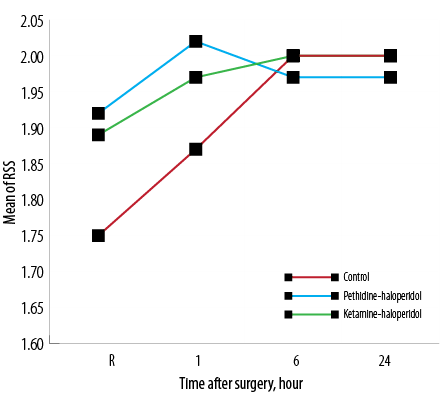
Figure 4: Average sedation rate of patients based on the Ramsay sedation scale (RSS) on arrival in the recovery room (R) and one, six, and 24 hours after surgery.
Discussion
Urinary catheterization is a necessary procedure in numerous lengthy surgeries, but many patients do not tolerate it, and it makes them agitated during the recovery period. We found that the incidence of CRBD after surgery was 55% in the control group and a combination of KH significantly reduced it to 17.9% in early recovery. The intensity of CRBD was significantly lower at one and six hours postoperatively. However, its beneficial effect no longer persisted at 24 hours.
Several researchers demonstrated the efficacy of ketamine as a preventive agent for CRBD.6–8 Results of this study are in concordance with the results reported by Moharari et al.6 They suggested that preemptive administration of IV ketamine (0.5 mg/kg) can reduce the incidence of CRBD near 30% in the early postoperative period (at 0 and one hour). However, no significant difference was observed between the two groups at the two- and six-hour evaluations.6
A systematic review and meta-analysis supported that ketamine, oxybutynin, and some anticholinergic drugs were useful in preventing CRBD.9
Haloperidol is a typical antipsychotic drug with some anticholinergic properties and was used as an adjuvant to potentiate preventive effects of ketamine and pethidine on CRBD.10 According to the results of this study, a combination of KH reduces the incidence of CRBD as much as 37%, which is greater than what other studies have ever found (oxybutynin (23% reduction), ketamine alone (30% reduction), and tolterodine (25% reduction)).6,11
In 1957, haloperidol, a dopamine D2 receptor antagonist was developed as a substitute derivative of meperidine, a phenylpiperidine analgesic.12 It has been suggested that D2 receptor antagonists if joined with opioids may enhance the analgesic effect of them.13
In this translational study, PH could not reduce the incidence and severity of CRBD. The result of our study shows that pethidine does not have a preemptive analgesic effect like ketamine. This difference may be explained that ketamine, which is a multipotential drug, exerts its analgesia more through N-methyl-D-aspartate (NMDA) receptor occupation and also has anti-inflammatory properties.14 It should be noted that the anticholinergic properties of pethidine have direct inhibitory effects on bladder contractions to reduce only CRBD symptoms. One study selected 98 patients undergoing elective abdominal, gynecologic, or orthopedic surgery. The participants received either 2 mg haloperidol or sterile water IV after the induction of anesthesia. The pain intensity and the demand for additional analgesics were measured in the sixth postoperative hour. They found that the pain scores in the haloperidol-treated patients were higher than the placebo group suggesting some anti-analgesic effect of haloperidol.15 The addition of ketamine to haloperidol might have deescalated the anti-analgesic effect of haloperidol while pethidine could not do the same as ketamine. There have been mixed observations of ketamine’s ability to provide preemptive analgesia. Although some researchers support the preemptive analgesic effects of ketamine, several others claimed no benefit.16 It should be noted that controversial findings might have been due to different doses of administered drugs or variable adjunct opioids in different studies, which may affect the role of the dominant receptor and subsequent clinical effect.
The intensity of surgical pain was not different between three groups during all study time points from recovery to 24 hours after surgery because we intentionally administered analgesics to relieve surgery associated pain.
The level of sedation was the third variable measured after surgery. Haloperidol is a sedative drug that we added to ketamine and pethidine; therefore, sedation score was a challenging variable for us in the postoperative period. We found no significant difference in the sedation score between three groups at any of the time points measured. Although both ketamine and pethidine on their own may exacerbate PONV, the incidence of PONV in both KH and PH groups were as low as the C group. We can explain this due to the anti-vomiting effect of haloperidol, which neutralized the emetic effects of both pethidine and ketamine.17 It is noteworthy that in a study by Moharari et al, which used ketamine alone, the incidence of PONV was high in the recovery room. In addition, the mean operation time in their study was lower than ours so this may have contributed to observing more adverse effects of ketamine in recovery.6
None of the patients had respiratory depression, hallucination, or unpleasant dreams. Due to the long operation times (which were much longer than the half-life of drugs), most of the injected drugs probably dissipated during the operation and had no effect in the recovery time. The beneficial effects seen in the KH group can be attributed to the preemptive effect of ketamine, which was injected just before the noxious stimulus (urinary catheterization).
Conclusion
The IV administration of low-dose ketamine 0.5 mg/kg plus haloperidol 0.04 mg/kg before urinary catheterization reduced the incidence and severity of CRBD at arrival in recovery and one and six hours after elective surgery for lumbar spinal stenosis while reducing the incidence of adverse effects.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
Authors would like to thank the research development center of Sina Hospital for their technical support.
references
- Binhas M, Motamed C, Hawajri N, Yiou R, Marty J. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Ann Fr Anesth Reanim 2011 Feb;30(2):122-125.
- 2. Kim HC, Lee YH, Jeon YT, Hwang JW, Lim YJ, Park JE, et al. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection: A double-blind randomised study. Eur J Anaesthesiol 2015 Sep;32(9):596-601.
- 3. Gupta D, Agarwal A, Dhiraaj S. Ketamine for treatment of catheter-related bladder discomfort. Br J Anaesth 2005 Nov;95(5):720.
- 4. Agarwal A, Dhiraaj S, Pawar S, Kapoor R, Gupta D, Singh PK. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg 2007 Nov;105(5):1454-1457.
- 5. Agarwal A, Yadav G, Gupta D, Singh PK, Singh U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study. Br J Anaesth 2008 Oct;101(4):506-510.
- 6. Shariat Moharari R, Lajevardi M, Khajavi M, Najafi A, Shariat Moharari G, Etezadi F. Effects of intra-operative ketamine administration on postoperative catheter-related bladder discomfort: a double-blind clinical trial. Pain Pract 2014 Feb;14(2):146-150.
- 7. Bai Y, Wang X, Li X, Pu C, Yuan H, Tang Y, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol 2015 Jun;29(6):640-649.
- 8. Akça B, Aydoğan-Eren E, Canbay Ö, Karagöz AH, Üzümcügil F, Ankay-Yilbaş A, et al. Comparison of efficacy of prophylactic ketamine and dexmedetomidine on postoperative bladder catheter-related discomfort. Saudi Med J 2016 Jan;37(1):55-59.
- 9. Hu B, Li C, Pan M, Zhong M, Cao Y, Zhang N, et al. Strategies for the prevention of catheter-related bladder discomfort: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016 Sep;95(37):e4859.
- 10. Dold M, Samara MT, Li C, Tardy M, Leucht S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst Rev 2015 Jan;1:CD009831.
- 11. Agarwal A, Dhiraaj S, Singhal V, Kapoor R, Tandon M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth 2006;96(3):377-380.
- 12. Creese I, Feinberg AP, Snyder SH. Butyrophenone influences on the opiate receptor. Eur J Pharmacol 1976 Mar;36(1):231-235.
- 13. Hagelberg N, Jääskeläinen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, et al. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol 2004 Oct;500(1-3):187-192.
- 14. Wang CQ, Ye Y, Chen F, Han WC, Sun JM, Lu X, et al. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 2017 Feb;343(343):30-38.
- 15. Ebneshahidi A, Akbari M, Mohseni M. Intraoperative haloperidol does not improve quality of recovery and postoperative analgesia. Adv Biomed Res 2013 Nov;2:85.
- 16. Becke K, Albrecht S, Schmitz B, Rech D, Koppert W, Schüttler J, et al. Intraoperative low-dose S-ketamine has no preventive effects on postoperative pain and morphine consumption after major urological surgery in children. Paediatr Anaesth 2005 Jun;15(6):484-490.
- 17. Büttner M, Walder B, von Elm E, Tramèr MR. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology 2004 Dec;101(6):1454-1463.