Toxic epidermal necrolysis (TEN), also known as Lyell’s syndrome, is a rare but severe mucocutaneous drug adverse reaction associated with high rate of mortality. Early and prompt detection of this condition, immediate withdrawal of the causative agent,1 and appropriate treatment plays a vital role in the patient’s survival. Drugs are the most common cause of TEN.2 Widespread epidermal sloughing occurs leaving an exposed dermis resulting in several complications. The mechanism of cell death is apoptosis via drug-induced CD8+ cell exocytosis of granzyme B/perforin and granulysin and through the activation of the Fas-Fas ligand pathway and tumor necrosis factor-alpha/death receptor pathway.3 For all cases, symptomatic and adequate supportive therapy is crucial, preferably in a burns unit.4,5 However, the use of specific therapy including systemic corticosteroids or intravenous immunoglobulin (IVIG) is still controversial.6,7 In some studies, corticosteroids are considered a life-saving therapy if given early during the disease course and in high doses,8 while other studies have shown that they increase the mortality rate and length of hospital stay.9,10 Several studies describe favorable outcomes following the use of IVIG11,12 in contrast to some studies, which reported poorer outcomes.7,13 However, the cost of IVIG is a major limiting factor.
Here we describe the case of a Filipino male patient with TEN who responded to combination therapy of low-dose IVIG and systemic corticosteroid.
Case Report
A 33-year-old Filipino male, not known to have any medical illnesses, working as a cook on a cruise ship from Dubai to Romania, was referred to Sultan Qaboos Hospital, Salalah, with a history of fever and rash lasting for three days. Fever was intermittent and high-grade and was relieved by analgesics given by the ship staff. The following day, the patient developed a non-itchy rash on his trunk and limbs.
The patient was admitted under the care of the medical team with a provisional diagnosis of dengue fever. However, the patient’s condition did not improve and got worse. Ten days after admission, he was referred to a dermatologist for a second opinion where he was diagnosed with TEN.
On examination, the patient was conscious and uncomfortable. Apart from a fever of 39.8°C and tachycardia of 114 beat/min, his other vital signs were normal. The skin on his trunk and limbs showed extensive blisters and denudation of the epidermis in sheets involving more than 70% of the body surface area [Figure 1]. Skin tenderness and Nickolsky's sign were positive. Bilateral conjunctival hemorrhage and purpuric eruption on lips were evident. Review of systems was unremarkable.
At the time of admission, his biochemical profile was within normal range except for C-reactive protein (CRP) 126.59 mg/L and alanine aminotransferase (ALT) 473 U/L. Later, the wound swab cultures showed Acinetobacter and Pseudomonas. The blood culture also showed Acinetobacter.
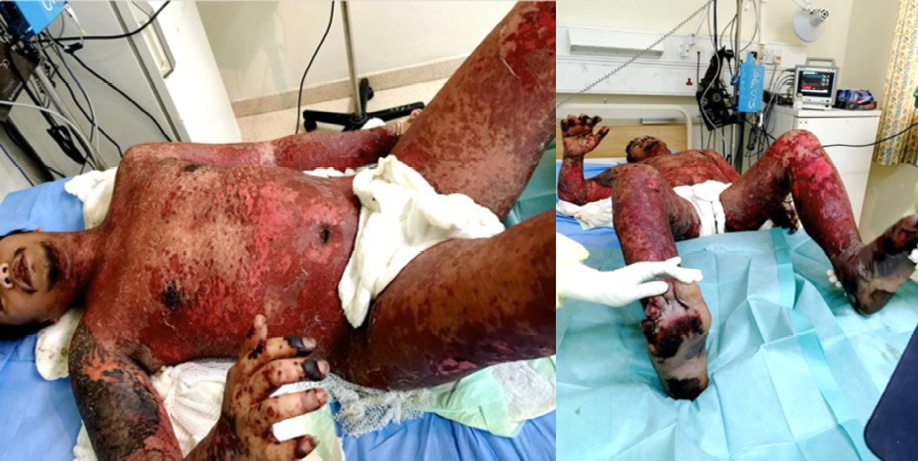
Figure 1: Denudation of the epidermis in sheets resembling wet cigar paper.
On day 10 of admission, the patient was immediately shifted to the burns unit for better supportive care. In addition, adjuvant systemic therapy of 0.1–0.3 mg/kg/day of dexamethasone for two consecutive days, 0.13 mg/kg for first day, and 0.1 mg/kg for the second day combined with 0.4 g/kg/day of IVIG for three days were given.
During the admission period, his condition was complicated by septicemia and disseminated intravascular coagulation, which were managed by broad-spectrum intravenous antibiotics according to culture and sensitivity and supportive treatment (anticoagulants, blood components, and antifibrinolytics).
Despite late initiation of treatment, the time for arresting disease progression and for reepithelialization was significantly short. The patient totally improved within two weeks without any sequelae [Figure 2].
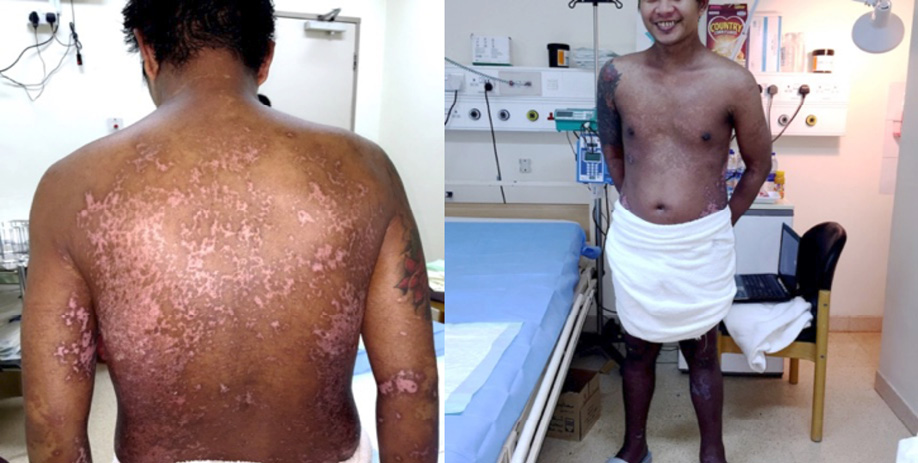
Figure 2: Patient’s skin condition on day 14 of treatment, healed totally with depigmented and hyperpigmented patches.
Discussion
TEN is a rare potentially fatal dermatologic disorder, usually caused by a reaction to drugs. The most common cause cited in the literature are sulfonamides, anticonvulsants, allopurinol, non-steroidal anti-inflammatory drugs, and lactam antibiotics (penicillin and cephalosporin).14 The distinctive feature of TEN is widespread epidermal separation due to keratinocyte apoptosis. Sepsis and multiorgan failure are the main causes of death in such patients.15
Apart from discontinuing the drugs that cause TEN,1 transferring the patient to a burns unit,4,5 and adequate supportive care, there is no consensus regarding specific TEN management strategies.16
Historically, systemic steroid was the first line of treatment for TEN; some studies showed that steroids are associated with increased mortality,9,10 higher rates of sepsis, and prolonged hospital stay.9 Nevertheless, mortality from steroid treatment was not detected in a recent retrospective case-control study from Europe.6 There has been interest in brief high-dose steroid therapy early during the disease course before significant epidermal loss.8
On the other hand, IVIG has recently become an important modality of treatment in TEN. Numerous studies describe the favorable outcomes following the use of IVIG.11,12 However, a meta-analysis including 17 observational studies did not find enough evidence to support IVIG use in patients with TEN.17 The exact effective dose of IVIG in TEN differs in various studies with most of them employing a total dose of 1.5–3.5 g/kg.18,19 However, few have reported improvement even with a low dose of 0.1–0.5 g/kg.20
In Oman, IVIG has been used in different centers for the management of TEN for the past decade. We use a total dose of 3 g/kg divided over three days and have found it useful in achieving early control of the disease despite many studies claiming otherwise.21
Furthermore, the effectiveness of combination therapy of IVIG and systemic steroids was evaluated in several studies. A recent meta-analysis of 26 studies showed that steroid and IVIG combination therapy has an impact in reducing recovery time compared with steroids alone. The favorable effects were greater in Asians and those who received a high dose of IVIG.22
The high cost of IVIG makes it difficult to obtain for most patients. Combination therapy with a cumulative low-dose IVIG (< 1.5 g/kg) and steroid is a novel cost-effective therapeutic option for TEN, which has been reported recently in one prospective comparative study conducted to find out the effectiveness of combination therapy of a low dose IVIG (0.2–0.5 g/kg) and rapidly tapering course of steroids (IV dexamethasone 0.1–0.3 mg/kg/day tapered in 1–2 weeks) versus steroid alone.23 The results supported the use of combination therapy as it was more effective in reducing mortality and accelerating disease resolution. The study hypothesizes that this combination may have a synergistic action targeting the different pathways of apoptosis and preventing disease progression earlier, therefore making the onset of reepithelialization faster and reducing the total steroid dose given earlier.23 Based on this study, we treated our patient with low-dose IVIG (0.4 g/kg/day for three days) combined with a corticosteroid (dexamethasone for two consecutive days, 0.13 mg/kg/day and then 0.1 mg/kg/day), and achieved excellent results. This case was considered the first in Oman and reported a great result with such treatment.
Conclusion
TEN is a rare, life-threatening disorder, generally induced by drugs, characterized by extensive necrolysis, and detachment of the epidermis. Many treatment modalities have been tried; however, optimal treatment guidelines are still unavailable. Corticosteroids have shown conflicting results, and for high-dose IVIG, the cost is a limiting factor. We achieved an excellent outcome using a new adjuvant treatment for TEN with a combination of low-dose IVIG and a short course of steroids. We suggest that it might be beneficial as well as cost-effective to use this modality of treatment, even in late stages of TEN. Additional randomized controlled trials are needed to confirm this finding. Future research on TEN should focus on adjuvant therapies to establish a standard treatment protocol.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000 Mar;136(3):323-327.
- 2. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008 Jan;128(1):35-44.
- 3. Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, et al. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol 1996 Apr;134(4):710-714.
- 4. McGee T, Munster A. Toxic epidermal necrolysis syndrome: mortality rate reduced with early referral to regional burn center. Plast Reconstr Surg 1998 Sep;102(4):1018-1022.
- 5. Palmieri TL, Greenhalgh DG, Saffle JR, Spence RJ, Peck MD, Jeng JC, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil 2002 Mar-Apr;23(2):87-96.
- 6. Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008 Jan;58(1):33-40.
- 7. Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003 Jan;139(1):33-36.
- 8. Herndon DN. Toxic epidermal necrolysis: a systemic and dermatologic disorder best treated with standard treatment protocols in burn intensive care units without the prolonged use of corticosteroids. J Am Coll Surg 1995 Mar;180(3):340-342.
- 9. Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg 1986 Nov;204(5):503-512.
- 10. Heimbach DM, Engrav LH, Marvin JA, Harnar TJ, Grube BJ. Toxic epidermal necrolysis. A step forward in treatment. JAMA 1987 Apr;257(16):2171-2175.
- 11. Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol 2012 Aug;167(2):424-432.
- 12. Al-Mutairi N, Arun J, Osama NE, Amr Z, Mazen AS, Ibtesam A, et al. Prospective, noncomparative open study from Kuwait of the role of intravenous immunoglobulin in the treatment of toxic epidermal necrolysis. Int J Dermatol 2004 Nov;43(11):847-851.
- 13. Brown KM, Silver GM, Halerz M, Walaszek P, Sandroni A, Gamelli RL. Toxic epidermal necrolysis: does immunoglobulin make a difference? J Burn Care Rehabil 2004 Jan-Feb;25(1):81-88.
- 14. Piechota M, Banach M, Kopeć A, Rysz J, Narbutt J. Toxic epidermal necrolysis (Lyell’ssyndrome): case report and review of the literature. Arch Med Sci 2008;4(4):480-485.
- 15. Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Stevens-Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 2008 Sep;7(8):598-605.
- 16. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007 Feb;56(2):181-200.
- 17. Tristani-Firouzi P, Petersen MJ, Saffle JR, Morris SE, Zone JJ. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin in children. J Am Acad Dermatol 2002 Oct;47(4):548-552.
- 18. Smith DI, Swamy PM, Heffernan MP. Off-label uses of biologics in dermatology: interferon and intravenous immunoglobulin (part 1 of 2). J Am Acad Dermatol 2007 Jan;56(1):e1-e54.
- 19. Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: an open uncontrolled study. Indian J Dermatol Venereol Leprol 2005 Nov-Dec;71(6):398-400.
- 20. Habib A, Pasha W, Raza N. Treatment of toxic epidermal necrolysis (TEN) with low dose intravenous immunoglobulin in child. J Coll Physicians Surg Pak 2010 Mar;20(3):205-207.
- 21. Al-Abri S, Al-Zadjali, Al-Reesi A. Multiple skin bullae. Oman Med J 2008 Ap;23(2): 118-119.
- 22. Ye LP, Zhang C, Zhu QX. The Effect of Intravenous Immunoglobulin Combined with Corticosteroid on the Progression of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Meta-Analysis. PLoS One 2016 Nov;11(11):e0167120.
- 23. Jagadeesan S, Sobhanakumari K, Sadanandan SM, Ravindran S, Divakaran MV, Skaria L, et al. Low dose intravenous immunoglobulins and steroids in toxic epidermal necrolysis: a prospective comparative open-labelled study of 36 cases. Indian J Dermatol Venereol Leprol 2013 Jul-Aug;79(4):506-511.