Beta-thalassemia, with a global carrier rate of 1.5%, equating to 80–90 million subjects and an estimated 50 000–60 000 new cases annually, is considered to be one of the most prevalent genetic diseases worldwide.1 β-thalassemia is characterized by inherited disorders in β-globin chain synthesis, which result in anemia due to hemolysis of red blood cells and erythroblasts as well as ineffective erythropoiesis.2–4 Given recent progress in the management of thalassemia patients, this group of patients’ survival and quality of life have increased significantly.5–7 However, hepatocellular carcinoma (HCC) has become the leading cause of mortality in thalassemia patients. In addition to hepatic iron overload and organ damage, a high prevalence of hepatitis C virus (HCV) infection among thalassemia patients is the major risk factor for the development of HCC.2,8
HCV is a small virus in the family Flaviviridae, with a single-stranded RNA genome and an enveloped icosahedral capsid. The genome encodes seven non-structural proteins (NS1, NS2, NS3, NS4A, NS4B, NS5A, and NS5B), a core protein, and two envelope glycoproteins (E1 and E2).9,10 Based on sequence analysis, there are seven genotypes and 67 subtypes of HCV.9 This virus is mainly transmitted through transfusion of blood and blood products, intravenous drug use, surgery, and tattooing.11,12 HCV infection is asymptomatic or acute hepatitis, which might clear or lead to chronic infection in approximately from 75% to 85% of the infected cases. Chronic HCV infection induces liver cirrhosis and HCC in a proportion of those chronically infected over time.13,14
Frequent blood transfusions increase the vulnerability of thalassemia patients to transfusion-mediated HCV infection. The high prevalence of HCV infection among thalassemia patients in different regions confirms this vulnerability.15,16 The prevalence of HCV among thalassemia patients depends on the levels of transfusion safety measures in the blood transfusion organizations.17 In Iran, despite the implementation of safe blood transfusion practices, the prevalence of HCV among the thalassemia population is considerably high.12 This high prevalence indicates deficiencies in the blood screening strategies. Therefore, information regarding the magnitude of HCV infection among thalassemia patients is of great importance for health care providers to assess blood safety and improve the quality of screening systems. However, despite this importance, no report on the epidemiology of HCV infection among thalassemia patients in the South of Iran is available. Therefore, the present study evaluated the prevalence, risk factors, and genotypic pattern of HCV infection among β-thalassemia patients in this region.
Methods
This descriptive-analytical cross-sectional study was conducted from March to June 2019. It included all patients with β-thalassemia major attending the transfusion center of the Bushehr University of Medical Sciences located in southern Iran. As a control group, 125 outpatients attending the university’s hospitals for blood tests were included in the study. The controls were matched in sex, age (± 3.0 years), and date of admission with the thalassemia patients and did not have a history of blood transfusion. All participants or the legal guardians of the minor subjects were requested to give written informed consent to screen their leftover serum samples for HCV infection and analysis. The sociodemographic characteristics and clinical information were obtained from each patient’s medical record at the transfusion centers. The Ethics Committee of the Bushehr University of Medical Sciences approved this study with reference number IR.BPUMS.REC.1395.183, and the Deputy Research and Affairs of the University funded this study with grant number 3225.
Serum samples of participants were tested for the presence of anti-HCV antibodies using commercially available an enzyme-linked immunosorbent assay (ELISA) kits (HCV Ab ELISA kit, DIA.PRO, Milan, Italy). Furthermore, the seropositive serum samples were tested for detecting HCV viremia and genotypes by semi-nested reverse transcriptase-polymerase chain reaction (RT-PCR), targeting the 5’UTR and core region of the genome, and sequencing as described previously.18,19 Following the extraction of HCV RNA from the samples using the High Pure Viral Nucleic Acid kit (Roche, Mannheim, Germany) and RT into cDNA using the SuperScript™ III cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA), the 680 bp length fragment of HCV genome was amplified in the first round PCR using outer primers AGCGTCTAGCCATGGCGT (–268 to –251) and ATGTACCCCATGAGGTCGGC (+410 to +391). The second-round PCR was performed using inner primers AGCGTCTAGCCATGGCGT (–268 to –251) and CACGTTAGGGTATCGATGAC (+383 to +364). The 580 bp length fragments from the second-round PCR were sequenced to determine HCV genotypes (Macrogen Co., Korea).
The data were analyzed by SPSS 17 package program (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.), and p-values < 0.05 were considered significant. The Student’s t-test was used to compare quantitative data between HCV-positive and HCV-negative thalassemia patients. Categorical data were analyzed by the Chi-squared test or Fisher’s exact test. Logistic regression analysis was used to determine the variables associated with the prevalence of HCV infection among thalassemia patients, and an odds ratio with 95% CI was determined.
Results
Serum samples were obtained from 125 patients with β-thalassemia major, including 91 patients from Bushehr, 15 from Borazjan, 10 from Delvar, six from Ahram city, and three from Kangan with ages ranging from 1 to 48 years (25.4±10.2). The majority of thalassemia patients were in the 20–29 years (39.2%) and 30–39 years (28.8%) age groups, respectively. Of the 125 thalassemia patients, 22 cases (17.6%; 95% CI: 11.9%–25.2%) were positive for anti-HCV antibodies. The controls were negative for anti-HCV antibodies. The highest rate of anti-HCV seropositivity was observed in the age group 30–39 years (33.3%) followed by the age group 20–29 years (18.4%), whereas the age groups < 10 years and > 39 did not show anti-HCV seropositivity. Overall, anti-HCV seroprevalence increased with age, so anti-HCV seropositive thalassemia patients had a significantly higher mean age (29.1±7.0) compared to anti-HCV seronegative thalassemia patients (24.6±10.6), (p = 0.049). Anti-HCV seroprevalence was higher among (18.1%) female thalassemia patients, (33.3%) residents of Kangan, (18.8%) Fars thalassemia patients, (18.1%) single patients, of patients with blood transfusion every two weeks (22.2%), diploma patients (27.9%), and thalassemia patients with alanine aminotransferase (ALT) levels of < 20 IU/L (21.3%) and aspartate aminotransferase (AST) levels of > 80 IU/L (42.9%). Nevertheless, anti-HCV seroprevalence among thalassemia patients was not statistically associated with gender distribution, place of residency, ethnicity, marital status, frequency of blood transfusion, and serum levels of ALT and AST [Table 1]. Notably, all anti-HCV seropositive thalassemia patients were negative for HBV and HIV.
Table 1: Prevalence of anti-hepatitis C virus (HCV) antibodies according to sociodemographic and quantitative variables among thalassemia patients in South of Iran.
|
Age groups, years
|
|
|
|
0.014
|
|
< 10
|
7 (5.6)
|
7 (100)
|
0 (0.0)
|
|
|
10–19
|
23 (18.4)
|
22 (95.7)
|
1 (4.3)
|
|
|
20–29
|
49 (39.2)
|
40 (81.6)
|
9 (18.4)
|
|
|
30–39
|
36 (28.8)
|
24 (66.7)
|
12 (33.3)
|
|
|
> 39
|
10 (8.0)
|
10 (100)
|
0 (0.0)
|
|
|
Gender
|
|
|
|
0.876
|
|
Female
|
72 (57.6)
|
59 (81.9)
|
13 (18.1)
|
|
|
Male
|
53 (42.4)
|
44 (83.0)
|
9 (17.0)
|
|
|
Place of residence, city
|
0.598
|
|
Bushehr
|
91 (72.8)
|
76 (83.5)
|
15 (16.5)
|
|
|
Borazjan
|
15 (12.0)
|
11 (73.3)
|
4 (26.7)
|
|
|
Delvar
|
10 (8.0)
|
8 (80.0)
|
2 (20.0)
|
|
|
Ahram
|
6 (4.8)
|
6 (100)
|
0 (0.0)
|
|
|
Kangan
|
3 (2.4)
|
2 (66.7)
|
1 (33.3)
|
|
|
Ethnicity
|
|
|
|
0.565
|
|
Fars
|
112 (89.6)
|
91 (81.3)
|
21 (18.8)
|
|
|
Afghan
|
3 (2.4)
|
3 (100)
|
0 (0.0)
|
|
|
Arab
|
10 (8.0)
|
9 (90.0)
|
1 (10.0)
|
|
|
Marital status
|
|
|
|
0.596
|
|
Single
|
116 (92.8)
|
95 (81.9)
|
21 (18.1)
|
|
|
Married
|
9 (7.2)
|
8 (88.9)
|
1 (11.1)
|
|
|
Frequency of blood transfusion
|
0.834
|
|
Once every two weeks
|
9 (7.2)
|
7 (77.8)
|
2 (22.2)
|
|
|
Once every three weeks
|
21 (16.8)
|
17 (81.0)
|
4 (19.0)
|
|
|
Once a month
|
62 (49.6)
|
51 (82.3)
|
11 (17.7)
|
|
|
As needed, every 35–50 days
|
6 (4.8)
|
6 (100)
|
0 (0.0)
|
|
|
Unknown
|
27 (21.6)
|
22 (81.5)
|
5 (18.5)
|
|
|
Education
|
|
|
|
0.029
|
|
Under diploma
|
66 (52.8)
|
60 (90.9)
|
6 (9.1)
|
|
|
Diploma
|
43 (34.4)
|
31 (72.1)
|
12 (27.9)
|
|
|
Upper diploma
|
16 (12.8)
|
12 (75.0)
|
4 (25.0)
|
|
|
ALT level, IU/L
|
|
|
|
0.846
|
|
< 20
|
47 (37.6)
|
37 (78.7)
|
10 (21.3)
|
|
|
20–40
|
19 (15.2)
|
17 (89.5)
|
2 (10.5)
|
|
|
41–80
|
21 (16.8)
|
17 (81.0)
|
4 (19.0)
|
|
|
> 80
|
9 (7.2)
|
8 (88.9)
|
1 (11.1)
|
|
|
Unknown
|
29 (23.2)
|
24 (82.8)
|
5 (17.2)
|
|
|
AST level, IU/L
|
|
|
|
0.359
|
|
< 20
|
36 (28.8)
|
29 (80.6)
|
7 (19.4)
|
|
|
20–40
|
23 (18.4)
|
19 (82.6)
|
4 (17.4)
|
|
|
41–80
|
30 (24.0)
|
27 (90.0)
|
3 (10.0)
|
|
|
> 80
|
7 (5.6)
|
4 (57.1)
|
3 (42.9)
|
|
Ab: antibodies; ALT: alanine aminotransferase; AST: aspartate aminotransferase.

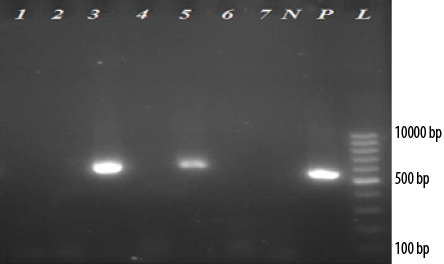 Figure 1: Electrophoresis of reverse transcriptase -polymerase chain reaction products of 5’UTR and core regions of (HCV) genome extracted from serum samples of thalassemia patients on 2.0% agarose gel. (L: 100-bp DNA ladder; P: Positive control; N: Negative control). Of 22 anti-HCV seropositive thalassemia patients, two cases (lanes 3 and 5) were positive for HCV (~580 bp). Lanes 1, 2, 4, 6, and 7 are negative for HCV patients.
Figure 1: Electrophoresis of reverse transcriptase -polymerase chain reaction products of 5’UTR and core regions of (HCV) genome extracted from serum samples of thalassemia patients on 2.0% agarose gel. (L: 100-bp DNA ladder; P: Positive control; N: Negative control). Of 22 anti-HCV seropositive thalassemia patients, two cases (lanes 3 and 5) were positive for HCV (~580 bp). Lanes 1, 2, 4, 6, and 7 are negative for HCV patients.
Of the 22 anti-HCV seropositive thalassemia patients, two (9.1%) had HCV viremia with genotype 3a. One of these cases was a 24-year-old woman with elevated levels of ALT and AST who had a blood transfusion every four weeks. This case was found to be positive in the first round of PCR. The second case was a 33-year-old man with normal levels of liver enzymes and blood transfusion every four weeks, which was positive in the second round of PCR. Overall, the prevalence of HCV viremia among thalassemia patients was 1.6% (95% CI: 0.5–5.6%). Electrophoresis of RT-PCR products of 5’UTR and core regions of HCV genome extracted from serum samples of thalassemia patients on 2.0% agarose gel is shown in Figure 1.
Discussion
HCV infection, with over 40% mortality in thalassemia patients, is considered a challenging transfusion-transmitted infection in Iran.20 In the absence of a prophylactic vaccine, the best way to control this disease is to improve blood transfusion safety. HCV prevalence rate in thalassemia patients can be used to evaluate the blood safety filters in different regions.17 Moreover, diagnosis and subsequent treatment of HCV-infected thalassemia patients can reduce the burden of HCV infection in the thalassemia population. Despite this importance, the epidemiology of HCV infection among thalassemia patients remains unknown in the South of Iran. Therefore, we evaluated the prevalence and genotypic distribution of HCV infection among patients with β-thalassemia major in this region and found HCV prevalence of 17.6% for anti-HCV antibodies and 1.6% for HCV viremia with genotype 3a.
The anti-HCV seroprevalence of 17.6% reported in the thalassemia patients is considerably higher than those reported among the general population (0.6%) and the blood donors (0.5%) of Iran.21,22 The anti-HCV seroprevalence observed in this study is higher than the anti-HCV seroprevalence of 0.1% reported in the blood donors of this region.23 Moreover, the controls were negative for anti-HCV antibodies. The control group was used to compare the prevalence of HCV infection between the multi-transfused thalassemia patients and the patients receiving no blood transfusion and evaluate the effect of blood transfusion on the prevalence of HCV infection. The results of this study indicate that multi-transfused patients, such as thalassemia patients, are at high risk for HCV infection. The high prevalence of HCV in this population is related to the transfusion of HCV-seronegative viremic blood units donated during the window period.12 Although screening of blood units based on detection of anti-HCV antibodies has significantly decreased the rate of HCV infection in patients with thalassemia in Iran,15,20 the current blood screening policy is inadequate to eliminate transfusion-transmitted HCV infection. Therefore, the blood screening strategies should be thoroughly reappraised, and sensitive molecular assays should be included in the screening process of donated blood for HCV infection in Iran.
The anti-HCV seroprevalence reported in this study is higher than those observed among thalassemia patients in the other parts of Iran, 3.4% in Tabriz (North-West of Iran),24 5.66% in Kurdistan (West of Iran),25 8% in Isfahan (Center of Iran),26 and 10.2% in Bandar Abbas (South-East of Iran),27 but lower than 18.8% in Gilan and Mazandaran (North of Iran),28 27.5% in Tehran (North-Center of Iran),29 and 28.1% in Khuzestan (South-West of Iran).30 According to a meta-analysis study, the overall seroprevalence of HCV among patients with thalassemia in Iran is 19%.15 High seroprevalence of HCV has been detected among thalassemia patients in Iraq (27.5%),31 Pakistan (29.4%),32 and Egypt (40.5%).33 While low seroprevalence of HCV has been reported in India (3.4%)34 and Bangladesh (13.51%).35 These studies show a wide range of variations in the geographical distribution of HCV infection, reflecting differences in the transfusion safety measures, the predominant risk factors, and the rates of HCV infection endemicity in different regions. As it is necessary to study the prevalence of HCV infection among blood donors to assess the current most common risk factors, the study of the epidemiology of HCV infection among thalassemia patients as blood recipients is also essential to evaluate the effectiveness of the transfusion safety measures employed in the blood transfusion organizations of the country. Therefore, these studies not only clarify the burden of HCV infection among thalassemia patients but are also essential for improving the current blood screening strategies to minimize the risk of transmitting HCV through blood transfusion.
In this study, anti-HCV seroprevalence was higher among female thalassemia patients and those patients with the highest frequency of blood transfusion. Moreover, those patients with abnormal levels of AST had higher HCV seroprevalence than thalassemia patients with normal levels of AST. However, neither gender nor frequency of blood transfusion and level of liver enzymes was associated with the seroprevalence of HCV among thalassemia patients. In addition, no significant association was found between HCV seroprevalence, place of residency, ethnicity, marital status, and level of education. In contrast, HCV seropositivity among thalassemia patients was statistically associated with age, so the highest rate of anti-HCV seroprevalence was observed in the age group 30–39 years compared to the other age groups (p = 0.014). Moreover, the age group > 39 did not show anti-HCV seropositivity. The probable reason for the absence of anti-HCV antibodies among the thalassemia patients aged > 39 years could be that the older thalassemia patients were infected years ago and, after recovery, their anti-HCV antibody titer decreased and reached an undetectable level over time. Notably, except for the age distribution, no risk factor was reported for HCV seroprevalence among thalassemia patients. In previous studies from Iran, HCV seroprevalence among thalassemia patients was significantly associated with the duration of blood transfusion.24–26,30 Similarly, some studies from Egypt and Iran have reported a significant association between the number of transfusions per month and HCV seropositivity.26,33 In contrast, some studies in Pakistan and Iraq demonstrated a higher seroprevalence of HCV among male thalassemia patients.32,36 In studies from Indonesia and Egypt, HCV seropositive thalassemia patients had higher levels of ALT and AST compared to HCV seronegative patients.37,38
In this study, 9.1% of anti-HCV seropositive thalassemia patients (2/22) had HCV viremia. Twenty anti-HCV seropositive thalassemia patients with negative HCV RNA results had past HCV infection and recovered as a result of antiviral therapy. Overall, 1.6% of the thalassemia patients (2/125) had HCV viremia, which is higher than that observed in the general population of Iran (0.4%).22 According to the previous studies from Iran, it can be concluded that from 1998 to 2017, the prevalence of HCV in thalassemia patients has decreased significantly due to the implementation of donor screening programs and the effective antiviral treatment by direct-acting antivirals (DAAs).15,20,39,40 So, the prevalence of HCV viremia among thalassemia patients in a study conducted in 2006–2007 was 22.3%30 compared to HCV viremia of 1.6% in the present
study in 2020.
The HCV genotype 3a was detected among thalassemia patients in this study. HCV genotype 3a reported in the present study follows the genotypic distribution of HCV in this region since the same genotypic pattern has been observed in previous studies.18,19 Infection with HCV genotype 3 is prevalent in intravenous drug users and is associated with advanced liver disease and cirrhosis and HCC progression.10 Therefore, prompt treatment of HCV-infected patients can prevent worse clinical outcomes. In addition to pathogenicity and clinical outcome of the infection, the response rate to antiviral therapy, type, and duration of treatment are associated with HCV genotypes.10,12 Patients with HCV genotypes 1 and 4 show lower response rates to interferon and ribavirin combination therapy. They need higher treatment duration than patients with HCV genotypes 2 and 3.10,41 With the development of DAAs-based therapy, treatment decision based on HCV genotype is less pronounced. Since DAAs often show pan-genotypic antiviral activity and use in combination therapy. Nevertheless, access to DAAs-based therapy is limited in low- and middle-income countries due to the high price and the restricted availability of DAAs.10,12,41
This study is the first report in South Iran that has determined the prevalence and genotype distribution of hepatitis C infection in β-thalassemia patients. In addition, participation of all β-thalassemia major patients' resident in Bushehr, Borazjan, Delvar, Ahram, and Kangan cities increases the generalizability of the results of this study. However, we did not investigate the effects of hepatitis C on the survival rates of HCV-infected thalassemia patients. As another limitation, other risk factors of HCV infection such as a history of surgery, dentistry, or tattoo as well as injecting drug abuse and other high-risk behaviors were not evaluated in this study due to the lack of information in records of the thalassemia patients.
Conclusion
This study reports the HCV prevalence of 17.6% for anti-HCV antibodies and 1.6% for HCV viremia with genotype 3a among β-thalassemia patients. These results reveal a high prevalence of HCV infection among β-thalassemia patients compared to the normal population, indicating ongoing HCV incidence among the thalassemia population in south Iran. Transfusion of HCV-seronegative viremic blood units donated during the seronegative window period contributes to HCV infection in thalassemia patients. Therefore, the determination of HCV infection in blood donors and recipients based on detecting HCV-RNA by PCR assay should be considered. The findings of this study highlight the need to include sensitive molecular assays in the screening process of donated blood for HCV infection
in Iran.
Disclosure
The authors declared no conflicts of interest. This study was funded by Bushehr University of Medical Sciences with grant number 3225.
Acknowledgments
The authors would like to acknowledge grant number 3225 supported by the Deputy Research and Affairs of the Bushehr University of Medical Sciences, Bushehr, Iran. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
references
- 1. Akhtar S, Nasir JA, Hinde A. The prevalence of hepatitis C virus infection in β-thalassemia patients in Pakistan: a systematic review and meta-analysis. BMC Public Health 2020 Apr;20(1):587.
- 2. Marsella M, Ricchi P. Thalassemia and hepatocellular carcinoma: links and risks. J Blood Med 2019 Sep;10:323-334.
- 3. Wali Y, Shidhani AA, Daar S. Agranulocyosis in beta thalassemia major patients treated with oral iron chelating agent (deferiprone). Oman Med J 2008 Oct;23(4):275-277.
- 4. Al-Khabori M, Daar S. Understanding iron metabolism: lessons from transfusion-dependent thalassemia. Oman Med J 2018 Jan;33(1):1-2.
- 5. Al-Moshary M, Al-Mussaed E, Khan A. Prevalence of transfusion transmitted infections and the quality of life in β-thalassemia major patients. Cureus 2019 Nov;11(11):e6129.
- 6. Daar S, Al Saadoon M, Wali Y, Al Mujaini R, Al Rahbi S, Chan MF, et al. Cognitive function in adults with beta-thalassemia major in Oman: a pilot study. Oman Med J 2021 Nov;36(6):e322.
- 7. Sadullah RK, Atroshi SD, Al-Allawi NA. Complications and challenges in the management of Iraqi patients with beta-thalassemia major: a single-center experience. Oman Med J 2020;35(4):e152.
- 8. Mangia A, Bellini D, Cillo U, Laghi A, Pelle G, Valori VM, et al. Hepatocellular carcinoma in adult thalassemia patients: an expert opinion based on current evidence. BMC Gastroenterol 2020 Aug;20(1):251.
- 9. Rabaan AA, Al-Ahmed SH, Bazzi AM, Alfouzan WA, Alsuliman SA, Aldrazi FA, et al. Overview of hepatitis C infection, molecular biology, and new treatment. J Infect Public Health 2020 May;13(5):773-783.
- 10. Taherkhani R, Farshadpour F. Global elimination of hepatitis C virus infection: progresses and the remaining challenges. World J Hepatol 2017 Nov;9(33):1239-1252.
- 11. Stasi C, Silvestri C, Voller F. Update on hepatitis C epidemiology: unaware and untreated infected population could fe the key to elimination. SN Compr Clin Med 2020;2(12):2808-2815.
- 12. Taherkhani R, Farshadpour F. Epidemiology of hepatitis C virus in Iran. World J Gastroenterol 2015 Oct;21(38):10790-10810.
- 13. Mahmoudvand S, Shokri S, Taherkhani R, Farshadpour F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J Gastroenterol 2019 Jan;25(1):42-58.
- 14. Shokri S, Mahmoudvand S, Taherkhani R, Farshadpour F, Jalalian FA. Complexity on modulation of NF-κB pathways by hepatitis B and C: a double-edged sword in hepatocarcinogenesis. J Cell Physiol 2019 Feb ;234(12).
- 15. Behzadifar M, Gorji HA, Bragazzi NL. The prevalence of hepatitis C virus infection in thalassemia patients in Iran from 2000 to 2017: a systematic review and meta-analysis. Arch Virol 2018 May;163(5):1131-1140.
- 16. Dumaidi K, Al-Jawabreh A, Samarah F, Rabayaa M. Prevalence of sero-molecular markers of hepatitis C and B viruses among patients with β-thalassemia major in Northern West Bank, Palestine. Can J Infect Dis Med Microbiol 2018;2018:1039423.
- 17. Shyamala V. Transfusion transmitted infections in thalassaemics: need for reappraisal of blood screening strategy in India. Transfus Med 2014 Apr;24(2):79-88.
- 18. Farshadpour F, Taherkhani R, Bakhtiari F. Prevalence and predominant genotype of hepatitis C virus infection and associated risk factors among pregnant women in Iran. Biomed Res Int 2021 Sep;2021:9294276.
- 19. Farshadpour F, Taherkhani R, Ravanbod MR, Eghbali SS. Prevalence and genotype distribution of hepatitis C virus infection among patients with type 2 diabetes mellitus. Med Princ Pract 2018;27(4):308-316.
- 20. Moghimbeygi M, Alavian SM. Eradication of hepatitis C virus infection in thalassemia patients in Iran using various treatment strategies. J Virus Erad 2020 Aug;6(3):100006.
- 21. Khodabandehloo M, Roshani D, Sayehmiri K. Prevalence and trend of hepatitis C virus infection among blood donors in Iran: a systematic review and meta-analysis. J Res Med Sci 2013 Aug;18(8):674-682.
- 22. Mirminachi B, Mohammadi Z, Merat S, Neishabouri A. Update on the prevalence of hepatitis C virus infection among Iranian general population: a systematic review and meta-analysis. Hepat Mon 2017;17:e42291.
- 23. Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and trends of transfusion-transmissible viral infections among blood donors in South of Iran: an eleven-year retrospective study. PLoS One 2016 Jun;11(6):e0157615.
- 24. Mirzaei G, Shamsasenjan K, Jafari B, Bagherizadeh Y, Sadafzadeh A, Bannazadeh-Baghi H, et al. Prevalence of HBV and HCV infection in beta-thalassemia major patients of Tabriz city, Iran. New Microbes New Infect 2021 Jul;43:100912.
- 25. Mohammadi S, Khodabandehloo M. Prevalence of hepatitis C virus antibodies among beta-thalassemia major patients in Kurdistan Province, Iran. Arch Clin Infect Dis 2017;12:e62419.
- 26. Ataei B, Hashemipour M, Kassaian N, Hassannejad R, Nokhodian Z, Adibi P. Prevalence of anti HCV infection in patients with Beta-thalassemia in isfahan-iran. Int J Prev Med 2012 Mar;3(Suppl 1):S118-S123.
- 27. Aminianfar M, Khani F, Ghasemzadeh I. Evaluation of hepatitis C, hepatitis B, and HIV virus serology pandemic in thalassemia patients of Shahid Mohammadi Hospital of Bandar Abbas, Iran. Electron Physician 2017 Mar;9(3):4014-4019.
- 28. Ghane M, Eghbali M, Abdolahpour M. Prevalence of hepatitis C amongst beta-thalassemia patients in Gilan and Mazandaran Provinces, 2011. Govaresh 2011;16:22-27.
- 29. Azarkeivan A, Toosi MN, Maghsudlu M, Kafiabad SA, Hajibeigi B, Hadizadeh M. The incidence of hepatitis C in patients with thalassemia after screening in blood transfusion centers: a fourteen-year study. Transfusion 2012 Aug;52(8):1814-1818.
- 30. Boroujerdnia MG, Zadegan MA, Zandian KM, Zandian KM. Prevalence of hepatitis-C virus (HCV) among thalassemia patients in Khuzestan Province, Southwest Iran. Pak J Med Sci 2009;25:113-117.
- 31. Abdullah Y, Hashim N, Sultan S. Incidence of hepatitis C infection among multi-transfused β-thalassemia major patients and its correlation to blood groups in Amara City. In: IMDC-SDSP 2020: Proceedings of the 1st international multi-disciplinary conference theme: sustainable development and smart planning, IMDC-SDSP 2020, Cyperspace. European Alliance for Innovation; 28-30 June 2020. p. 449.
- 32. Yasmeen H, Hasnain S. Epidemiology and risk factors of transfusion transmitted infections in thalassemia major: a multicenter study in Pakistan. Hematol Transfus Cell Ther 2019 Oct - Dec;41(4):316-323.
- 33. Mansour AK, Aly RM, Abdelrazek SY, Elghannam DM, Abdelaziz SM, Shahine DA, et al. Prevalence of HBV and HCV infection among multi-transfused Egyptian thalassemic patients. Hematol Oncol Stem Cell Ther 2012;5(1):54-59.
- 34. Sabat J, Dwibedi B, Dash L, Kar SK. Occult HBV infection in multi transfused thalassemia patients. Indian J Pediatr 2015 Mar;82(3):240-244.
- 35. Bhuyan GS, Noor AU, Sultana R, Noor FA, Sultana N, Sarker SK, et al. Frequency of hepatitis B, C and HIV infections among transfusion-dependent beta thalassemia patients in Dhaka. Infect Dis Rep 2021 Jan;13(1):89-95.
- 36. Raham TF, Wahed SSA and Alhaddad HN. Prevalence of Hepatitis C among patients with βthalasemia in Diyala-Iraq. J tech 2011;24(4):113-120.
- 37. Wahidiyat PA, Liauw F, Adnani NB, Putriasih SA. Prevalence of hepatitis and its correlation with serum ferritin and aminotransferase levels among thalassemia major patients in Indonesia. Paediatr Indones 2017;57:177.
- 38. Salama KM, Ibrahim OM, Kaddah AM, Boseila S, Ismail LA, Hamid MM. Liver enzymes in children with beta-thalassemia major: correlation with iron overload and viral hepatitis. Open Access Maced J Med Sci 2015 Jun;3(2):287-292.
- 39. Takhviji V, Azizi E, Kordian A. Hepatitis C virus prevalence among patients with thalassemia and inherited bleeding disorders in Iran: a systematic review and meta-analysis. Hematol Transfus Int J 2018;6:163-169.
- 40. Jafroodi M, Davoudi-Kiakalayeh A, Mohtasham-Amiri Z, Pourfathollah AA, Haghbin A. Trend in prevalence of hepatitis C virus infection among β-thalassemia major patients: 10 years of experience in Iran. Int J Prev Med 2015 Sep;6:89.
- 41. Elzuoki AN, Elzouki I, Albarassi S, Gammo M, Burwaiss A. Hepatitis C genotypes in Libya: correlation with patients’ characteristics, level of viremia, and degree of liver fibrosis. Oman Med J 2017 Sep;32(5):409-416.