Neonatal jaundice is usually a self-resolving incident and is found in 60% of normal newborns typically ending 72 to 96 hours after birth.1 Jaundice (hyperbilirubinemia) is as a result of increased bilirubin concentrations beyond the normal levels and evident with yellowish pigmentation in the skin, conjunctiva, and other mucous membranes.
In healthy individuals, the concentration of bilirubin in blood plasma is negligible (< 17 µmol/L); however, this is increased in person’s suffering from jaundice (> 30 µmol/L). The concentration of bilirubin in newborns increases immediately after birth due to high hemoglobin turnover, which the body unable to eliminate as quickly as needed. This is seen in approximately 60% of term infants and 80% of preterm infants.2 If untreated, this could lead to serious brain damage and even result in death. Therefore, it is imperative to have an early diagnosis. Treatment usually involves light therapy, and if the condition becomes severe, blood transfusion.3,4
Physicians or nursing staff would order serum bilirubin tests in neonates based on visual evaluation. Visual inspection of the skin or sclera is an instant and cost-free way of estimating the serum bilirubin though not sufficiently accurate, particularly when screening newborns belonging to diverse ethnicity or variegated cultural backgrounds.5,6 Transcutaneous spectrophotometric measurement or transcutaneous bilirubinometry is an alternative way of estimating serum bilirubin and is non-invasive, fast, and relatively inexpensive.1,7 Transcutaneous bilirubinometry testing has been widely accepted among non-white babies over visual assessment due to the recognized limitations of visual inspection.
In neonates, the gold standard to measure hyperbilirubinemia continues to be only total serum bilirubin (TSB) which requires a heel-prick blood sample from the patient. This technique is painful, and if performed frequently, could lead to anemia. The accuracy of clinical laboratory methods is affected while measuring transcutaneous bilirubin (TcB) in newborns as the blood collected is frequently hemolyzed. Frequent measurement of TSB in infants with bilirubin concentrations below the treatment threshold is inappropriate because it requires unnecessary blood sampling. Moreover, anticipating results also delays the discharge of both the mother and baby from the hospital.8,9
Optical methods present a non-subjective assessment. In neonates, the most feasible alternative for screening clinically significant hyperbilirubinemia is the use of a transcutaneous bilirubinometer. As a result, to support non-invasive diagnosis of hyperbilirubinemia and approximation of TSB levels, various methods have been developed. This include cephalocaudal staging of jaundice and TcB reference devices.10
We sought to determine the accuracy of two distinct non-invasive methods for bilirubin assessment (TcB using BiliChek and optical imaging (ToB) using BiliCapture) [Figure 1], compared with a laboratory hematology analyzer as the reference method. We also sought to determine whether ToB measurements alter clinical practice.
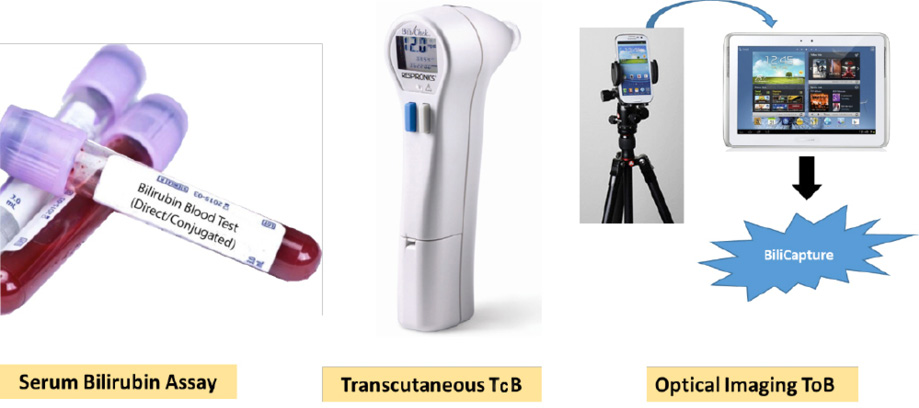
Figure 1: Different techniques used for measuring the level of bilirubin-serum bilirubin in the laboratory using blood serum, transcutaneous bilirubinometry using BiliChek, and optical imaging using BiliCapture.
Methods
We collected patient samples from June 2015 to December 2016 from the King Khalid University Hospital at Al-Majma’ah and Riyadh (Eastern Province), Saudi Arabia and Alpine Hospital, Gurgaon, India.
Parents of all neonates were provided with written information leaflets and a briefing of the advantages and benefits of the study to them as well as to the community. Thereafter, written consent was obtained from those parents who agreed to participate in the study. Before commencing the study, both Ethics and Research and Development committee’s approvals (MUREC-Jan.05/COM-2015) were obtained after being classified as a non-risk study according to the principles from the Declaration of Helsinki. All data and information were kept confidential and used only for this study.
The exclusion criteria were gestational age (GA) less than 35 weeks, body weight less than 2 kg, major disorder developed during neonatal intensive care unit or hospital admission, and discharge before 18 hours post-delivery. Medical records of mothers and the first-trimester ultrasound examination were employed for determining GA. GA was assessed by the Ballard Scale if the recorded data in the medical records were falsified.5,11
The sample size was calculated using the following formula:12
|
n = |
p (1 – p) (Zβ + Zα/2)2 |
|
(p1 – p2)2 |
Where p1 and p2 are the anticipated proportions = 20% and 50%; p1–p2 is the difference between proportions = 30%; Z1–β is the desired power of
study = 90% (1.28); Z1-α/2 is the desired level of significance = 5% (1.96); p is the average of proportions = 0.35; the alternative hypothesis is one-sided. The calculated sample size was 47, and a sample size of 50 neonates was used for the study.
We assessed healthy newborns (n = 50) in this cross-sectional study. If their serum bilirubin level was greater than 12.9 mg/dL, they were identified as having hyperbilirubinemia.
Blood samples of 5 mL were taken from the maternal side of the umbilical cord immediately after delivery and sent to the laboratory. The sample was processed for separation of serum within two hours of collection and refrigerated at 2–8 °C until the serum bilirubin measurement was completed.
Diazo method with dichloroaniline (DCA) was used for estimation of TSB.13 BiliChek (Philips Respironics, Murrysville, PA, USA) and BiliCapture were tested on each infant without any previous information. Bilirubin measurement was performed initially using BiliChek on the forehead and BiliCapture to obtain conjunctival images under controlled artificial light.
Capillary blood samples were used for universal predischarge screening of TSB. These samples were taken by piercing the newborn’s heel. Using UNISTAT® Bilirubinometer (Reichert Technologies, USA) and direct spectrophotometric assay with an accuracy (bias) of ±5% TSB measurements were accomplished.14 Skilled personnel from the clinical chemistry laboratory of the hospital made all the required measurements.
A simultaneous TcB measurement using BiliChek was taken every time a clinical evaluation was made to obtain TSB measurement. Based on the TSB result, the decision was taken by the clinicians to commence phototherapy. GA was taken into consideration for calculation of the bilirubin concentration threshold for starting the phototherapy and calculated as: threshold = (GA × 10) – 100. Accordingly, the threshold for a 35-week gestation infant would be 250 mmol/L. Infants who did not require phototherapy were monitored clinically and based on clinical grounds the medical and/or nursing staff would decide whether to repeat TSB. However, if the baby required phototherapy, the TSB was repeated after 6–8 hours and 12–24 hours to ensure a descending trend and correction in bilirubin levels until a decision was taken to end the phototherapy. To be certain that the bilirubin levels were still within the normal range and below the treatment level and there was no significant rebound increase in its level, TSB was repeated 8–12 hours after stopping phototherapy. BiliChek TcB measurement was assessed five-times in succession in 10 infants to assess precision.9
TcB measurement was also carried out within 15–30 minutes by applying BiliChek (Respironics Inc., Chichester, UK) to the infant’s forehead with the infant lying supine whenever the samples for TSB measurement were collected. Before each TcB measurement, the disposable probe tips were calibrated as per the manufacturer’s direction. Following calibration, the tips were applied with gentle pressure to the skin and the BiliChek device was spontaneously activated for five repeated spectral analyses, demonstrating a calculated averaged reading in either mmol/L or mg/dL.
During/after phototherapy, if the neonate required repetitive TSB measurement simultaneous multiple measurements were also done. The region of forehead utilized for TcB was covered and not exposed to direct sunlight and/or phototherapy. Before initiating phototherapy for infant, a phototherapy protective patch (BilEclipseTM, Philips Respironics, Murrysville, PA, USA) was positioned over the site of measurement.
BiliChek measurement was done while all phototherapy lights were turned off. After taking the measurement the protective patch was opened and the flap was secured before resuming phototherapy. Once the neonate was withdrawn from phototherapy, the BilEclipse flap was detached. However, subsequent BiliChek measurements were obtained from the site on the forehead that had not been exposed to the phototherapy lights. Skin areas with bruising, birthmarks, hematomas, or excessive hair were carefully spotted and avoided. Recording of GA, birth weight, postnatal age, and ethnicity was done after.
BiliCapture optical imaging was done after TSB and TcB. The conjunctival image was captured using Samsung 10 × zoom camera. This image was then shared on Samsung tablet with the BiliCapture software installed. With the help of this software, the particular area of interest in the eye showing more yellow color was demarcated and later on estimated for the bilirubin value.15
Statistical calculations and graphs were prepared with the help of Excel (Microsoft Office Professional 2010) and SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Mean±SD were reported for normally distributed quantitative variables, median and interquartile range were reported for non-normally distributed variables, and number and percentages were reported for qualitative variables. The normality was assessed by the Kolmogorov-Smirnov (K-S) test. A Pearson’s correlation coefficient (r) was calculated to determine the overall level of agreement between the two methods and the standard reference bilirubin values. Bland and Altman analysis was also used since it determines bias, precision, and agreement between optical measurement and transcutaneous bilirubinometry, comparing with the automated analysis in the laboratory as the reference.16 A p-value < 0.050 was considered statistically significant, and all p-values were two-tailed.
Outliers were checked based on Clinical and Laboratory Standards Institute guidelines EP6-A.17 Bland-Altman plots were used to identify mean bias (the average of the differences between measurements obtained from the two assays being compared) and 95% limits of agreement between methods. Agreement in the assessment of bilirubin levels between assays was assessed using Cohen’s Kappa (agreement: poor = < 0.40, fair to good = 0.40–0.75, excellent = > 0.75).
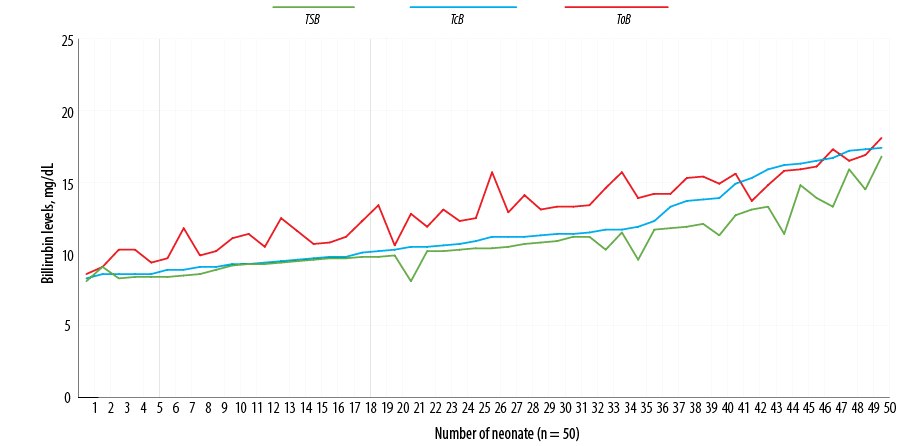
Figure 2: Distribution of total serum bilirubin (TSB), transcutaneous bilirubin (TcB), and optical imaging bilirubin (ToB) for the study population.
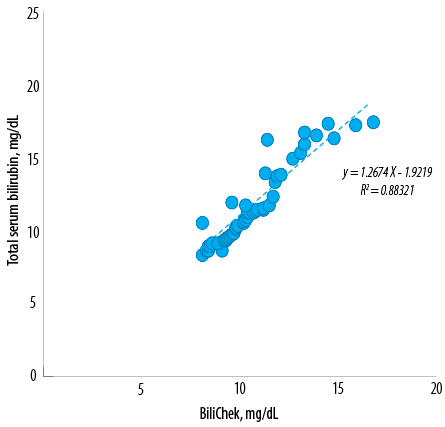
Figure 3: Linear regression between neonatal total serum bilirubin and transcutaneous bilirubin concentrations. A second order polynomial equation was derived from the correlation between them with r2 = 0.88. The lower end of a confidence interval was 0.623 and the upper end was 0.770.
Results
Fifty term or near-term infants (28 male and 22 female) were nominated with a mean GA of 39.0 weeks (range = 35–42 weeks). The mean age of neonate at the time of blood sampling was three days (range = 0–5 days), and the mean birth weight was 3.3 kg (range = 2.16–4.35 kg) [Table 1]. Medical conditions such as sepsis, respiratory distress, or cardiac or circulatory disease were not present in these infants.
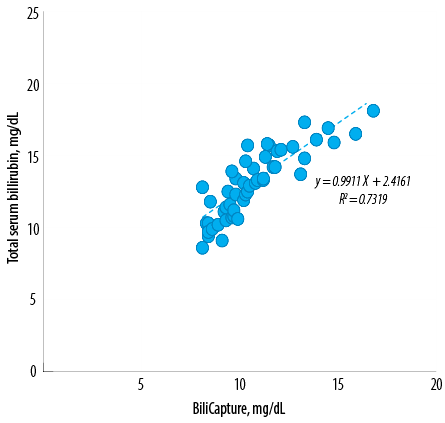
Figure 4: Comparison of neonatal total serum bilirubin and optical imaging concentrations. A second order polynomial equation was derived from the correlation between them with r2 = 0.73. The lower end of a confidence interval was 0.609 and the upper end was 0.868.
The mean serum bilirubin concentration for all infants was 10.7±2.0 mg/dL; the range was 8.1 to 16.8 mg/dL. The mean bilirubin concentration obtained using BiliChek was 11.6±2.7 mg/dL (range = 8.3–17.4 mg/dL). The mean bilirubin concentration measured through optical imaging using BiliCapture was 13.1±2.3 (range = 8.6–18.1 mg/dL) [Figure 2].
Linear correlation existed between TSB and TcB values (y = 1.27×–1.92, r² = 0.88, standard error of the estimate (sy.x) = 0.74) [Figure 3]. The specificity and sensitivity of the transcutaneous bilirubinometer was calculated using a value of > 25 as a cut-off point. This cut-off point was determined by evaluating the data in accordance with guidelines given in the transcutaneous bilirubinometer manual. This same cut-off point was also obtained by calculating the value that gave approximately 25 false-positive results for every one false-negative result given the prevalence of hyperbilirubinemia in this study. The device categorized hyperbilirubinemia with a sensitivity of 92.0% and a specificity of 75.6%. This represented one false-negative value and 20 false-positive values. The predictive value of a positive test was 47.0%. The predictive value of a negative test was 99.0%.
A linear relationship also existed between TSB and ToB values (y = 0.99× + 2.42, r² = 0.73, sy.x = 0.47) [Figure 4]. Using a BiliCapture value of > 4 as a cut-off point, the device categorized hyperbilirubinemia with a sensitivity of 88.0% and specificity of 76.0%. Four out of five false-negative results occurred in the first few measurements, the remaining measurements with the BiliCapture produced a sensitivity of 95.0% and specificity of 81.0%. Predictive values of positive and negative tests were 41.0% and 94.0%, respectively (n = 100). The mean bilirubin values for TSB, TcB, and ToB are presented in Table 2.
Correlation and regression analyses were utilized to determine the statistical significance between bilirubin levels obtained from the optical imaging device BiliCapture. The Bland-Altman method was used for evaluating the agreement between the conventional laboratory serum detection and transcutaneous bilirubinometer BiliChek and our optical imaging device, BiliCapture.18,19 To determine whether a new method can be used interchangeability with an already established method, the limit of agreement between them and its clinical evaluation are the crucial factors. In line to the methodology given by Bland and Altman, we assessed our proposed non-invasive bilirubin detection method to an established biochemical method to find statistical agreement [Table 2]. A good agreement with the comparison values for the two non-invasive instruments Bland-Altman plots [Figure 5], showed slopes of the regression lines closed to 1.0 for all methods tested.
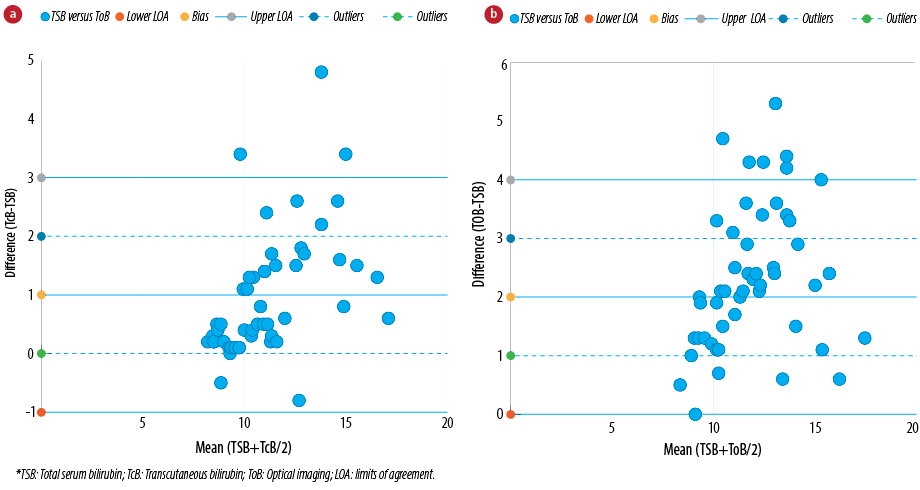
Figure 5: Bland and Altman representation of comparative analysis between (a) neonatal serum and transcutaneous bilirubin concentrations (BiliChek) and (b) neonatal serum and optical imaging bilirubin concentrations (BiliCapture). Bias (dashed line), limits of agreement (bias±1.96* standard deviation (SD), continuous lines) and outlier limits (bias±1 mg/dL, dotted lines) are represented on the graphs (n = 50). The mean difference between the two methods is depicted as a horizontal line and is rated as bias. The other two horizontal lines (mean±2SD) represent limits of agreement which explains that 95% of the differences were assumed to lie within these limits.
Non-invasive TcB measurements are a desirable modality since it is a quick, non-invasive technique to screen for hyperbilirubinemia.6 According to National Institute of Clinical Excellence guidelines (2018), TcB was acclaimed as a substitute to TSB for inspecting jaundiced neonates.2 The approach of transcutaneous bilirubinometry has become predominantly widespread among health care professionals engaged in neonatal care because it reduces invasive blood sampling and permits a time effective and reasonably precise estimation of bilirubin levels.20 Evidence-based data is lacking regarding the use of TcB measurements in preterm infants, especially those receiving phototherapy.21
In 1980, TcB using a bilirubinometer was first introduced for non-invasive determination of skin bilirubin in neonates.22 This bilirubinometer was developed by Minolta and utilized a two-filter design to determine “the yellow color of skin”. Subsequently other commercial devices were developed, for example, ColorMate TLc-BiliTest System which involves an preliminary measurement shortly after birth, BiliChek (by SpectRx)23 which requires five repeated measurements at one site and is built on reflectance analysis at various wavelengths, and Jaundice Meter (by Minolta/Hill-Rom Air-Shields) which is based on two wavelength analyses.
In BiliChek, the use of multiple wavelength readings permits (400 to 760 nm) adjustment for differences in skin pigmentation and hemoglobin, reducing the need for a patient-specific baseline reading. An investigation performed to compare BiliChek and Jaundice Meter JM-102 established that the BiliChek system was less erratic than the Jaundice Meter, and that the BiliChek system was not influenced by skin color which purports that a multi-wavelength analysis offers significantly better results for evaluation of skin bilirubin.9,24 The BiliChek device displayed more accurate data than clinical laboratory bilirubin measurements obtained from HPLC.23 While BiliChek was accepted as a significant enhancement over the previous transcutaneous devices,1 a clean, disposable tip is essential for each measurement, considerably increasing the cost of the test.
A recent study discovered that TcB measurements using BiliChek reproduce the same information as obtained from capillary plasma bilirubin concentration of < 240 mmol/L (< 14 mg/dL).25 Beyond this concentration, they assumed that the BiliChek device should be considered only for screening the samples and later it should be referred to central laboratory for validation. Another study indicated that BiliChek should not be chosen as a substitute for TSB, even though they discovered that TcB was effective in the detection of infants with a TSB 300 μmol/L (17.5 mg/dL).26 These infants demand additional bilirubin monitoring and repeatedly receive phototherapy.
Our study has confirmed that the conjunctiva may be a target organ for the diagnosis of jaundice independent of race, age, and sex by means of a simple diffused reflection measurement technique. Based on the principle above, we have also advanced a non-invasive, easy, expeditious, reliable, and practical device for routine measurement of bilirubin levels.
The results obtained from both methods employed in our study had exceptional strong correlations within the level of bilirubin in the laboratory. Therefore, the mean values of bilirubin obtained from both BiliChek and BiliCapture was believed to be close to the “true” bilirubin concentration and provided as a comparison value, since a true standard test for bilirubin determination has not been recognized. Only a few reference methods have been offered and published which are neither used commonly nor accepted extensively.
Our study had several limitations. First, the mechanism and etiology of hyperbilirubinemia were not determined. Second, the number and percentage of neonates who developed significant hyperbilirubinemia and required hospital admission was low (n = 5, 1.5%). As such, a small sample size was the main limitation of this study. It is a very difficult and sensitive issue to obtain blood from neonates as most parents are reluctant to give consent for the procedure. Another limitation of our study was that it was conducted in confined settings involving single hospital units. This study also had an ethnic limitation as the samples were from two different countries. The present method of measuring bilirubin through optical imaging involves capturing conjunctival images, which might be a limiting factor for the accuracy of bilirubin levels.
It is indispensable to have a vague and sensitive mechanism to make a fair and valid comparison between new tools and more traditional “gold standard” reference practices, for performing the studies and analyzing data related to neonatal hyperbilirubinemia. Our findings prove that ToB compared to TcB is a non-invasive method to determine neonatal hyperbilirubinemia and is significantly economical without wasting time. However, the understanding of the precision of a new device is vital before accepting it into clinical practice and using it for significant therapeutic interventions. ToB is an alternative aid for immediate diagnosis of hyperbilirubinemia especially in outpatient follow-up, which can help to prevent consequential outcomes.
The authors declared no conflicts of interest. The study was funded by the Basic Health and Science Research Center (BHSRC).
The authors would like to thank BHSRC for their financial support as well as routine supervision of the research project.