Many conditions of short stature are classified as idiopathic due to the inability to identify the underlying etiology.1,2 Families of children with idiopathic short stature (ISS) seek medical intervention, and growth hormone (GH) therapy is often requested. Several studies have assessed the efficacy and safety of GH therapy in ISS over the past two decades. Nonetheless, the use of GH treatment for this indication is still debatable. This issue, along with the high cost of GH treatment,2,3 has resulted in a denial of health insurance coverage in many regions, including the United Arab Emirates (UAE).
We sought to evaluate the effect of GH therapy in ISS patients in our region and compare it to the effect seen in patients with GH deficiency (GHD), the first and most commonly approved indication for GH. Given the high rate of consanguinity in our local society4 and its potential effect on the underlying etiologies of ISS, we assumed that the outcome of GH treatment in our patient population with ISS might be different from what has been reported in other cohorts.3,5,6 This is the first study to assess the outcome of GH therapy on final adult height in patients with ISS in UAE and Gulf region.
Methods
We conducted a retrospective chart review of all patients 3–15 years of age who were on GH treatment and were followed-up regularly in the pediatric endocrine clinic of our facility between 1 January 2005 and 31 December 2013. Since our hospital exclusively provides GH in our region, we used the pharmacy record to identify all patients.
Patients were diagnosed with ISS when their height (Ht) Z-score was < -2 standard deviation score (SDS), and insulin-like growth factor-1 (IGF-1) and IGF-binding protein 3 (IGF-BP3) were within the normal age and gender-matched range, plus one or more of the following criteria:
- Peak GH response ≥ 10 mcg/L on a standard, two stimulants GH provocative test.
- Confirmed family history of ISS in one sibling.
- Non-delayed bone age.
Patients were excluded if they took medications known to affect growth (such as steroids or immune-suppressants), had chronic systemic comorbidities, and/or did not maintain regular follow-ups.
The diagnosis of GHD was established when the peak response to two stimulants GH provocative
test was < 10 mcg/L in children with congenital or acquired pituitary disorder, Ht Z-score < -2SDS, or confirmed low growth velocity (GV).
Table 1: Comparison of the baseline characteristics and the change in height (Ht) Z-score between patients in the ISS and GHD group.
|
Age, median (IQR), years |
12.4 (10.4–13.6) |
10.6 (7.5–12.0) |
0.060 |
|
Tanner scale I or II, % |
81.0 |
96.0 |
1.000 |
|
Baseline Ht Z-score |
-2.7 ± 0.6 |
-2.5 ± 0.9 |
0.110 |
|
Ht Z-score after 12 months |
-2.2 ± 0.7 |
-1.9 ± 0.8 |
0.320 |
|
Final Ht Z-score, n |
-1.4 ± 0.5 (15) |
-1.5 ± 1.0 (18) |
0.600 |
|
∆Ht 0-12 |
0.5 ± 0.3 |
0.5 ± 0.4 |
0.540 |
|
ISS: idiopathic short stature; GHD: growth hormone deficient; FH: final height; IQR: interquartile range.. |
The effect of GH therapy was evaluated by calculating the difference in Ht Z-score after 12 months (ΔHt 0-12) to determine the short-term value and upon reaching final height (ΔHt 0-FH) for the long-term value. FH was assumed when the GV was equivalent to < 2 cm/year, and bone age was greater than 14 years in females and 16 years in males. We also compared the ΔHt 0-12, and ΔHt 0-FH between patients with ISS patients and those with GHD (idiopathic or secondary to a congenital or acquired pituitary disorder).
Data were analyzed using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Height was expressed as mean (SD). Other characteristics were expressed as median and interquartile range (IQR). Baseline characteristics were analyzed using the independent sample t-test for continuous data and chi-square test for nominal data. Auxological characteristics were analyzed using the paired t-test or independent sample t-test, as appropriate. Comparison between height SDS differences (ΔHt SDS) at different durations of GH treatment within the groups was done using the general linear model (repeated measure ANOVA). The assumptions of normality, sphericity, and homoscedasticity were met. ΔHt SDS at different durations between patients with ISS and patients with GHD were tested with multivariate analysis. Statistical significance was considered at p-value ≤ 0.050.
The study was approved by Al Ain Medical District Human Research Ethics Committee – Protocol No. 13/50 (CRD 256/13).
Results
Between 2005 and 2013, 21 patients were diagnosed with and treated for ISS while 29 patients were treated for GHD of different etiologies. Out of 21 ISS patients, nine were females and 12 were males; 12 were Emiratis. The median (IQR) age patients started GH treatment was 12.4 (10.4–13.6) years, and the median bone age was 10.6 (7.5–12.0) years. Fifteen patients were followed until they reached final adult height.
The ΔHt 0-12 improved from -2.7 (0.6) SDS to -2.2 (0.7) SDS (p < 0.001), and the ΔHt 0-FH in the subgroup of patients who reached FH also improved from -2.7 (0.6) SDS to -1.3 (0.5) SDS (p < 0.0010) [Figure 1].

Figure 1: Height (Ht) gain at 12-months and final height (FH) in patients with idiopathic short stature.
A total of 29 patients (14 females, 15 males; out of 29 patients, 20 were Emiratis) had GHD, 18 of whom reached final adult height. Table 1 summarizes the comparison between the two groups. Patients in the ISS group had a higher median age and fewer were prepubertal compared to the GHD group; however, the difference was not statistically significant for either factor.
There was no statistical significance in the ΔHt 0-12 between the two groups (p = 0.540). However, ΔHt 0-FH was significantly higher in ISS group, p = 0.050 [Figure 2].
When we segregated patients with idiopathic GHD (n = 8), we found no significant difference in ΔHt 0-FH, 1.3 (0.5) SDS in ISS, and 1.2 (0.8) SDS in idiopathic GHD, p = 0.710.
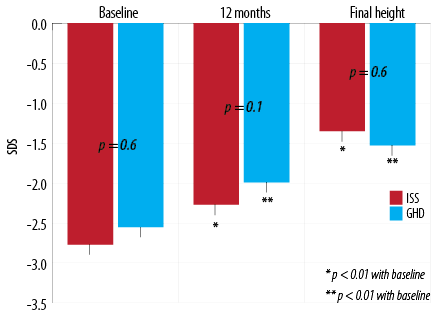
Figure 2: Change in standard deviation score (SDS) from baseline to 12-months post-treatment in patients with idiopathic short stature (ISS) and growth hormone deficient (GHD).
Discussion
To the best of our knowledge, this is the first study that looked at the long-term effect of GH therapy in patients with ISS in the UAE and Gulf Region.
Short stature is one of the most common complaints that present to pediatric endocrinologists. The underlying etiologies cannot be identified occasionally,2,7 and the condition is listed as familial short stature and/or constitutional growth delay.1,2,8 It is estimated that 60–80% of short children who are at or below -2 SDS are diagnosed as ISS.7
In spite of the US Food and Drug Administration approval of GH therapy for the treatment of ISS in 2003, the decision to use GH for this indication continues to be an area of debate due to the variable outcome findings9 and the high cost of treatment, which can exceed $35 000 per inch gained in adult height.2,3
We conducted this retrospective cohort study to assess the effect of GH therapy in patients with ISS in our community, which has a high rate of consanguinity. We found that GH improved the Ht Z-score significantly in the first year of treatment, and the effect was maintained, and even improved, toward adult height. While our findings are similar to those of Sotos et al,6 they are clearly better than what has been reported in several other cohorts internationally.3,5,10,11 This difference might be attributed, at least partially, to a consanguinity-related higher prevalence of genetic conditions that have a better response to GH therapy in our local population. Future genetic work-up on cases of ISS in children of consanguineous couples might shed more light on this area.
Unlike other studies,12,13 we found that the impact of GH treatment on FH was higher in patients with ISS compared to GHD of all etiologies. This was despite the fact that patients with GHD were younger and higher percentage were prepubertal at the commencement of treatment, two factors that significantly affected the outcome of GH treatment.2,13–15 This result might be because some patients with GHD had underlying etiologies that led to an earlier suspicion and diagnosis of their GH status, those patients were started on GH therapy at an earlier stage and a relatively better height SDS hence, the gain in height was less noticeable. Another possibility is that more than half of this cohort had multiple pituitary hormonal deficiencies, and they were less compliant taking their multiple medications, including the daily administration of GH. Additionally, they were more predisposed to the potential effect of the other comorbidities on the normal growth process. To further investigate this point, we compared ΔHt 0-FH between the subgroup of patients with idiopathic (isolated) GHD and those with ISS and found no difference.
Few studies have shown that there is a correlation between the response to treatment in the first year, and the achieved FH,5,13,16,17 and our results were consistent with those findings. In spite of the relatively advanced age of our patients when they were started on GH treatment, the improvement in height SDS exceeded the suggested value of a successful treatment of 0.3–0.5 SDS,2,13 while the average used dose of GH was similar to that used in other studies which reported a dose-dependent response to GH in patients with ISS.6,8–14,16
The main limitations of this study are the sample size, the lack of control group, and the retrospective design.
Conclusion
Our study indicates that GH treatment in children with ISS has a positive impact on FH, which is comparable to the effect seen in children with GHD. This result may impact on the clinical practice and health policies in our region.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P. Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Horm IGF Res 2008 Apr;18(2):89-110.
- 2. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al; 2007 ISS Consensus Workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008 Nov;93(11):4210-4217.
- 3. Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis. Arch Pediatr Adolesc Med 2002 Mar;156(3):230-240.
- 4. al-Gazali LI, Bener A, Abdulrazzaq YM, Micallef R, al-Khayat AI, Gaber T. Consanguineous marriages in the United Arab Emirates. J Biosoc Sci 1997 Oct;29(4):491-497.
- 5. Hughes IP, Harris M, Choong CS, Ambler G, Cutfield W, Hofman P, et al; Australasian Paediatric Endocrine Group (APEG). Growth hormone regimens in Australia: analysis of the first 3 years of treatment for idiopathic growth hormone deficiency and idiopathic short stature. Clin Endocrinol (Oxf) 2012 Jul;77(1):62-71.
- 6. Sotos JF, Tokar NJ. Growth hormone significantly increases the adult height of children with idiopathic short stature: comparison of subgroups and benefit. Int J Pediatr Endocrinol 2014;2014(1):15.
- 7. Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr 1994 Jul;125(1):29-35.
- 8. Pedicelli S, Peschiaroli E, Violi E, Cianfarani S. Controversies in the definition and treatment of idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol 2009;1(3):105-115.
- 9. Deodati A, Cianfarani S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: systematic review. BMJ 2011 Mar;342:c7157.
- 10. Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al; National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2004 Jul;89(7):3140-3148.
- 11. Al-Abdulrazzaq D, Al-Taiar A, Hassan K, Al-Basari I. Recombinant growth hormone therapy in children with short stature in Kuwait: a cross-sectional study of use and treatment outcomes. BMC Endocr Disord 2015 Dec;15:76.
- 12. Kim SA, Choe YR, Yang EM, Kim CJ. Comparison of growth hormone treatment in patients with idiopathic short stature and idiopathic growth hormone deficiency. Chonnam Med J 2014 Aug;50(2):63-66.
- 13. Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al; KIGS International Board. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res 2007;68(2):53-62.
- 14. Lee PA, Sävendahl L, Oliver I, Tauber M, Blankenstein O, Ross J, et al. Comparison of response to 2-years’ growth hormone treatment in children with isolated growth hormone deficiency, born small for gestational age, idiopathic short stature, or multiple pituitary hormone deficiency: combined results from two large observational studies. Int J Pediatr Endocrinol 2012 Jul;2012(1):22.
- 15. Ismail NA, Metwaly NS, El-Moguy FA, Hafez MH, El Dayem SM, Farid TM. Growth response of Egyptian children with idiopathic short stature during four years of growth hormone therapy. Indian J Hum Genet 2011 Sep;17(3):218-225.
- 16. Kriström B, Dahlgren J, Niklasson A, Nierop AF, Albertsson-Wikland K. The first-year growth response to growth hormone treatment predicts the long-term prepubertal growth response in children. BMC Med Inform Decis Mak 2009 Jan;9:1.
- 17. Hopwood NJ, Hintz RL, Gertner JM, Attie KM, Johanson AJ, Baptista J, et al. Growth response of children with non-growth-hormone deficiency and marked short stature during three years of growth hormone therapy. J Pediatr 1993 Aug;123(2):215-222.