Among the leading causes of sepsis in very low birth weight (VLBW) infants are systemic fungal infections (SFIs). Candida-attributable mortality has been estimated in the range of 25–55%; survivors may experience adverse long-term neurodevelopmental outcomes.1–3 SFI was implicated in about 12% of all late-onset sepsis cases among VLBW infants.4
Mucosal and skin fungal colonization is an important risk factor for SFI. Candida spp. is a common colonizing organism due to its ability to adhere to the human epithelium, particularly in the gastrointestinal (GI) tract. Most infants admitted to the neonatal intensive care unit (NICU) become rapidly colonized by Candida; the most frequent sites during the first two weeks of life are the GI and respiratory tracts. Candida infection may be transmitted either from the mother during birth or as a nosocomial acquisition in the NICU.5,6 In addition, preterm infants’ immature humoral and cell-mediated immunity, weaker immune responses, and need for prolonged treatments, enable the growth and spread of fungal infections.
Other risk factors include the presence of central catheters, intubation, prolonged parenteral feeding, delayed enteral feeding, broad-spectrum antibiotics, and certain medications such as corticosteroids, theophylline, and histamine type 2 receptor blockers.4,7–9 SFI in VLBW infants is often reported from the second to sixth weeks after birth. It is not confined to hematogenous sepsis. SFI may affect the central nervous system (meningitis and brain abscesses), urinary tract, and soft and deep tissues. SFI may also cause endocarditis, endophthalmitis, hepatitis, and pneumonitis.10
Invasive fungal disease in VLBW infants can be prevented and treated by interrupting the process of colonization, for which oral nonabsorbable antifungal agents such as nystatin and intravenous fluconazole are generally used.11 In cases of infection by non-albicans Candida species with reduced susceptibility to fluconazole, the alternative is usually nystatin. This study aimed to compare the efficacies of two prophylactics, systemic fluconazole, and oral nystatin, in preventing Candida colonization in high-risk infants.
Methods
This randomized controlled clinical trial was conducted in Al Zahra Hospital (a tertiary referral teaching hospital in North Western Iran) from March 2020 to April 2021. The ethics committee of Tabriz University of Medical Sciences approved the study (Ref: IR.TBZMED.REC.1400.144). The study is registered in the Iranian Registry of Clinical Trials as No. IRCT20210908052412N1.
To estimate the required sample size, we used the prevalence rates reported in a Turkish study by Aydemir et al,12 who found a Candida colonization rate of 11.7% in the group treated with nystatin and 10.8% in the group treated with fluconazole. Setting statistical power at 80% and significance (alpha) at 0.05, we arrived at a minimum required sample size of 31 in each group.
Inclusion criteria were inborn preterm infants with gestational age < 32 weeks and birth weight < 1500 g, who were managed at NICU. Exclusion criteria were major congenital anomalies, hemodynamic instability in the first two days of life, elevated (twice the upper normal limits) levels of serum glutamic oxaloacetic transaminase and serum glutamate pyruvate transaminase in the first 72 hours of life, as well as parental refusal.
After obtaining informed written consent from parents, we enrolled 120 neonates in this study. The `sealed envelope' method was used to randomly allocating them to two groups A and B of 60 neonates. This maintained the integrity of the random allocation process. Figure 1 shows the flow diagram of the study methodology including the selection process.
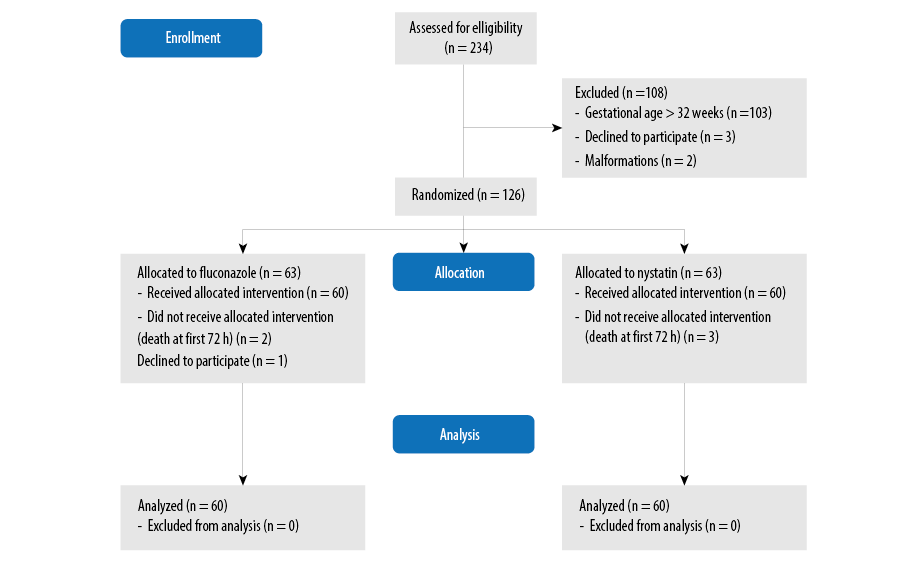 Figure 1: Flow diagram of the study methodology.
Figure 1: Flow diagram of the study methodology.
Patients in group A received 3 mg/kg fluconazole intravenously twice weekly from the first 72 hours of life which was continued for six weeks or till discharge from NICU. In cases where the intravenous line was discontinued during NICU stay, the treatment was switched to oral fluconazole 6 mg/kg twice weekly. In patients in group B, oral nystatin 100 000 unit (1 mL) was given every eight hours, from the first 72 hours of life till six weeks or discharge from NICU. The rest of the management protocols — such as respiratory management and total parenteral nutrition — were the same in both groups. The primary endpoint of the study was the comparative incidence and the associated mortality rate of SFI in each group during hospitalization. The secondary endpoint was the comparative complications of prematurity that arose in each group.
Bronchopulmonary dysplasia (BPD) was diagnosed when a preterm infant required supplemental oxygen during the initial 28 postnatal days, and it was further classified at 36-week postmenstrual age according to the degree of oxygen supplementation.13 Intraventricular hemorrhage (IVH) was diagnosed by cranial ultrasound examination which was performed in all infants at days 5–7 by an experienced pediatric radiologist. Retinopathy of prematurity (ROP) was diagnosed by an expert ophthalmologist by indirect ophthalmoscopic eye examination from age 4–6 weeks after birth.
Liver function tests (aspartate aminotransferase, alanine transaminase, alkaline phosphatase, and total and direct bilirubin), complete blood count, blood urea nitrogen, and creatinine were tested weekly during the anti-fungal prophylaxis. Liver failure was defined as liver enzymes or conjugated hyperbilirubinemia > 5-fold the upper limit of normal.
All demographic, laboratory, and clinical data were recorded by an experienced nurse who was not aware of the study’s objective and patient grouping.
Statistical analysis was performed using SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Quantitative data was presented as mean±SD and qualitative data as frequency and percentage. An independent t-test was used for testing continuous normally distributed data. Categorical data was compared between groups using the chi-square or Fisher 's exact test. Two-tailed tests were used and a p-value < 0.05 was considered statistically significant.
Results
The subjects of this study were 120 preterm neonates (53 girls and 67 boys), the mean gestational age was 28.2±1.4 weeks. The demographic characteristics of the patients are shown in Table 1. The most common maternal complication was preeclampsia. Intubation and mechanical ventilation were performed in 18 (15.0%) neonates of whom 10 (16.7%) were from group A. The interventions and procedures in the patients are shown in Table 2.
Table 3 lists groupwise details of the SFI incidence during hospitalization. Also given are the complications of prematurity arising in each group.
Table 1: Demographic and other characteristics of preterm infants (N = 120).
|
Gestational age, weeks, mean±SD
|
28.4 ± 1.4
|
28.0 ± 1.5
|
0.451
|
|
Birth weight, grams, mean±SD
|
1169 ± 125
|
1145 ± 114
|
0.296
|
|
Male
|
38 (63.3)
|
29 (48.3)
|
0.141
|
|
Mean Apgar score
|
7.0 ± 0.7
|
7.1 ± 0.6
|
0.458
|
|
Delivery mode, cesarean section
|
54 (90.0)
|
47 (78.3)
|
0.089
|
|
Intrauterine growth restriction
|
15 (25.0)
|
17 (28.3)
|
0.394
|
|
Maternal diabetes mellitus
|
3 (5.0)
|
5(8.3)
|
0.485
|
|
Preeclampsia
|
28 (46.7)
|
32 (53.3)
|
0.361
|
|
Prolonged rupture of membranes
|
4 (6.7)
|
3 (5.0)
|
0.569
|
|
Oligohydramnios
|
3 (5.0)
|
1 (1.7)
|
0.279
|
|
Antenatal corticosteroid
|
|
|
0.078
|
|
Nil
|
20 (33.3)
|
20 (33.3)
|
|
|
One dose given
|
15 (25.0)
|
30 (50.0)
|
|
|
Two doses given
|
25 (41.7)
|
10 (16.7)
|
|
Table 2: Medical management details of preterm infants (N = 120).
|
Surfactant therapy
|
57 (95.0)
|
55 (91.7)
|
0.445
|
|
Mechanical ventilation
|
10 (16.7)
|
8 (13.3)
|
0.653
|
|
Peripherally inserted central catheter
|
26 (43.3)
|
25 (41.7)
|
0.488
|
|
Umbilical vein catheter
|
26 (43.3)
|
25 (41.7)
|
0.488
|
|
Intralipid
|
41(68.3)
|
40 (66.7)
|
0.806
|
|
Urinary catheter
|
14 (23.3)
|
11 (18.3)
|
0.339
|
*Significance.
Table 3: Primary and secondary endpoints in preterm infants (N = 120).
|
Systemic fungal infection
|
3 (5.0)
|
3 (5.0)
|
0.999
|
|
Retinopathy of prematurity
|
1 (1.7)
|
4 (6.7)
|
0.045*
|
|
Intraventricular hemorrhage
|
7 (11.7)
|
3 (5.0)
|
0.025*
|
|
Bronchopulmonary dysplasia
|
12 (20.0)
|
20 (33.3)
|
0.145
|
*Significance.
Table 3 reveals that SFI was detected as fungal urinary infection in six (5.0%) infants with three cases from each group. Severe stages of ROP occurred in four (6.7%) infants in group B and one (1.7%) in group A (p = 0.045). Intraventricular hemorrhage was detected in brain ultrasound examination in three (5.0%) neonates in group B and seven (11.7%) in group A (p = 0.025). No case of necrotizing enterocolitis (NEC) or liver failure was reported in the entire cohort. Three deaths were reported overall, none of them attributable to Candida infection.
Discussion
In our study, the rates of SFIs and mortality were similar among preterm infants with fluconazole or nystatin prophylaxis. Among the complications of prematurity, advanced stages of ROP and BPD were significantly more common in group B, whereas IVH was significantly more common in group A.
Our findings were similar to those of Aydemir et al,12 who compared intravenous fluconazole and oral nystatin with placebo in a cohort of 278 VLBW infants. Nystatin prophylaxis resulted in decreased Candida colonization in the GI tract, skin, and respiratory tract. Invasive Candida infections were significantly lower in the fluconazole and nystatin groups compared with the placebo group (3.2%, 4.3% vs. 16.5%; p < 0.001). However, unlike their study, we did not have a placebo group. Several other studies, including a meta-analysis, also found nystatin to be as effective as fluconazole in preventing SFI.14–17
Rundjan et al,17 compared the fungal colonization and SFI among 95 preterm infants of whom 47 received oral nystatin and 48 received placebo. The incidence of fungal colonization was 29.8% among infants in the nystatin group and 56.3% in the control group. In addition, five cases of SFI were reported, all of which were in the placebo group. They concluded that using nystatin showed a protective effect against SFI among VLBW preterm infants without significant differences.
In our study, three (5.0%) cases developed SFI, which was less than the results of a meta-analysis that demonstrated one in every 4–9 infants receiving either fluconazole or nystatin prophylaxis was prevented from developing SFI. The lower gestational age of our cohort may explain the difference.16
No significant adverse effects have been reported in infants treated with fluconazole prophylaxis such as bacterial infections, NEC, focal bowel perforation, and cholestasis.18–21 Because of the very high osmolality of oral suspension of nystatin (3002 mOsm/L), there is a concern with the use of this hyperosmolar medication and the risk of NEC. Like us, Aydemir et al,12 found no significant differences in the rates of NEC (8.6%, 9.6%, 9.9%) or mortality (8.6%, 8.5%, 12.1%) in fluconazole, nystatin, and placebo groups, respectively.
Some evidence favors fluconazole for antifungal prophylaxis owing to its greater efficacy of 80–90% compared to that of nystatin prophylaxis (50–60%), its relative safety in VLBW infants, twice-weekly administration, intravenous administration in the case of GI disease or hemodynamic instability, and probably lower cost. Although the cost per dose of nystatin is lower, the total cost of a full course of nystatin prophylaxis, administered more than once daily, will exceed that of fluconazole, administered only twice a week.
The potential harms of routine antifungal prophylaxis include Candida resistance patterns, drug side effects, and cost. Limiting the prophylactic use of fluconazole to high-risk preterm infants may help delay the development of fungal resistance to antifungal drugs. Some studies recommended fluconazole prophylaxis be limited to extremely LBW (ELBW) infants, presence of central line catheters, broad-spectrum antibiotics use, and NICUs with moderate-to-high incidence of invasive candidiasis.19,22
In our study, the rates of BPD and severe ROP were significantly lower in the fluconazole group than in the nystatin group. A recent Chinese study by Zhang et al,23 compared the complications of prematurity among preterm infants treated with fluconazole prophylaxis and those with nosocomial fungal infection. They reported significantly lower BPD, ROP needing interventions, and periventricular leukomalacia/IVH in the prophylaxis group with no increased risks of serious short-term adverse side effects. A meta-analysis by Wang et al,24 also found that the prophylactic use of fluconazole had no significant role in the occurrence of common complications in preterm infants.
A 2014 study found no benefit in giving antifungal prophylaxis to extremely ELBW infants, defined as weighing below 750 g.25 More recently, a meta-analysis of studies on fluconazole prophylaxis in ELBW infants weighing below 1000 g found significantly decreased colonization of Candida spp. and lower mortality in six of the seven studies reviewed.26
The limitations of this study were its small sample size, lack of long term follow-up of the studied patients, and lack of fungal colonization detection.
Conclusion
The oral nystatin and intravenous fluconazole were similarly effective with respect to SFI prevention in our study. Future multicenter studies with large number of patients recommended establishing the appropriate antifungal prophylaxis for best outcome.
Future studies with a larger number of patients are recommended before routine administration of nystatin prophylaxis.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We thank the NICU nurses involved in the care of the
studied infants.
references
- 1. Adams-Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al; Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr 2013 Oct;163(4):961-967.e3.
- 2. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al; National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004 Nov;292(19):2357-2365.
- 3. Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al; National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006 Jan;117(1):84-92.
- 4. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics 2002 Aug;110(2 Pt 1):285-291.
- 5. Ali GY, Algohary EH, Rashed KA, Almoghanum M, Khalifa AA. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. J Matern Fetal Neonatal Med 2012 Jun;25(6):789-795.
- 6. Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol 2003 Oct;27(5):357-364.
- 7. Barton M, O’Brien K, Robinson JL, Davies DH, Simpson K, Asztalos E, et al. Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infect Dis 2014 Jun;14:327.
- 8. Cook A, Ferreras-Antolin L, Adhisivam B, Ballot D, Berkley JA, Bernaschi P, et al. Neonatal invasive candidiasis in low- and middle-income countries: data from the NeoOBS study. Med Mycol 2023 Mar;61(3):myad010.
- 9. Filioti J, Spiroglou K, Roilides E. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Med 2007 Jul;33(7):1272-1283.
- 10. Noyola DE, Fernandez M, Moylett EH, Baker CJ. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis 2001 Apr;32(7):1018-1023.
- 11. Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 2012 Dec;18(Suppl 7):38-52.
- 12. Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed 2011 May;96(3):F164-F168.
- 13. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001 Jun;163(7):1723-1729.
- 14. Mersal A, Alzahrani I, Azzouz M, Alsubhi A, Alsawaigh H, Albshri N, et al. Oral Nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: non-inferiority trial. J Clin Neonatol 2013 Apr;2(2):88-92.
- 15. Violaris K, Carbone T, Bateman D, Olawepo O, Doraiswamy B, LaCorte M. Comparison of fluconazole and nystatin oral suspensions for prophylaxis of systemic fungal infection in very low birthweight infants. Am J Perinatol 2010 Jan;27(1):73-78.
- 16. Austin N, Cleminson J, Darlow BA, McGuire W. Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database Syst Rev 2015 Oct;2015(10):CD003478.
- 17. Rundjan L, Wahyuningsih R, Oeswadi CA, Marsogi M, Purnamasari A. Oral nystatin prophylaxis to prevent systemic fungal infection in very low birth weight preterm infants: a randomized controlled trial. BMC Pediatr 2020 Apr;20(1):170-179.
- 18. Kaufman DA. Getting to zero: preventing invasive Candida infections and eliminating infection related mortality and morbidity in extremely preterm infants. Early Hum Dev 2012;88 Suppl 2:S45-S49.
- 19. Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 2001 Dec;345(23):1660-1666.
- 20. Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics 2008 Apr;121(4):703-710.
- Cleminson J, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev 2015 Oct;2015(10):CD003850.
- Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al; Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med 2007 Jun;356(24):2483-2495.
- 23. Zhang D, Xie D, He N, Wang X, Dong W, Lei X. Prophylactic use of fluconazole in very premature infants. Front Pediatr 2021 Oct;9:726769.
- Wang XL, MaY, Wang SH, Dong WB, Lei XP. A meta-analysis of fluconazole for the prevention of invasive fungal infection in preterm infants. Am J Transl Res 2021;13 (2):434-447.
- 25. Benjamin DK Jr, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, et al; Fluconazole Prophylaxis Study Team. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA 2014 May;311(17):1742-1749.
- 26. Robati Anaraki M, Nouri-Vaskeh M, Abdoli Oskoei S. Fluconazole prophylaxis against invasive candidiasis in very low and extremely low birth weight preterm neonates: a systematic review and meta-analysis. Clin Exp Pediatr 2021 Apr;64(4):172-179.