Coronary Artery Fistula with Heart Failure in Early Infancy
Salim Al-Maskari, Prashanth Panduranga, Abdulla Al-Farqani
doi:10.5001/omj.2010.73
ABSTRACT
Heart failure in early infancy is commonly caused by lesions leading to pulmonary over circulation secondary to left-to-right shunt. This case report describes an unusual cause of significant left-to-right shunt in a 2 months old infant presenting with congestive heart failure, which was diagnosed with transthoracic echocardiography. In this infant, transthoracic echocardiography with Doppler color flow mapping allowed direct visualization of a large right coronary artery to right ventricular fistula that was surgically corrected successfully. From the 1Department of Cardiology, Royal Hospital, Muscat, Oman.
Received: 03 Jun 2010
Accepted: 27 Jun 2010
Address correspondence and reprint request to: Dr. Prashanth Panduranga, Department of Cardiology, Royal Hospital, Post Box 1331, Muscat-111, Sultanate of Oman
E-mail: prashanthp_69@yahoo.co.in
INTRODUCTION
Heart failure in early infancy is commonly caused by left-to-right heart shunts. Such lesions include ventricular septal defects, atrioventriculoseptal defects, complex congenital heart disease with unrestricted pulmonary blood flow, etc. This case report describes an unusual cause of significant left-to-right shunt in an infant presenting with congestive heart failure, which was diagnosed with transthoracic echocardiography.
CASE REPORT
A 2 month old full-term female infant, born without complications presented to Royal hospital with congestive heart failure precipitated by chest infection. She was tachypneic with intercostals and subcostal recession. She was pink, had full volume bounding pulses and femoral pulse was easily felt. Her precordium was hyperdynamic and there was grade 3/6 continuous murmur in the left lower sternal border. Her liver was 4.5 cm below the costal margin. An electrocardiogram was normal for her age, and chest radiograph showed cardiomegaly and plethoric lungs. Transthoracic echocardiography showed situs solitus with mildly dilated right ventricle (RV) and normal left ventricle.
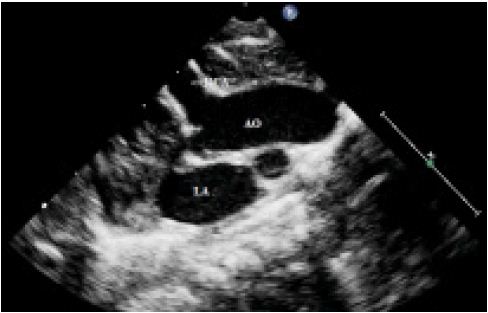
There was a 9 mm secundum atrial septal defect with left to right shunt, normal pulmonary venous drainage into the left atrium, intact interventricular septum, non-dilated coronary sinus with no persistent ductus arteriosus (PDA) and normal valves. There was gross dilatation (7 mm) of the right coronary artery (RCA) throughout the whole course along the RV free wall up to apex where it was seen draining into the RV with a 4 mm single exit orifice. (Figs. 1 & 2A)
Color Doppler revealed turbulent blood flow starting from RCA, traversing lateral aspect of RV and finally drained into the RV cavity with Doppler interrogation demonstrating a continuous flow signal, (Fig. 2B). There was no localized aneurysmal formation noted. The calculated pulmonary to systemic blood flow (Qp/Qs) ratio was 2.1:1 and the pulmonary artery systolic pressure was 55 mmHg.
Figure 1: Transthoracic echocardiography showing grossly dilated right coronary artery in an infant with coronary-cameral fistula.
LA=Left atrium; AO=Aorta; RCA=Right coronary artery.
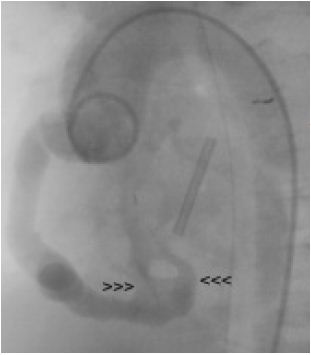
The infant was treated with diuretics and antibiotics, but needed ventilator management. She underwent cardiac catheterization to delineate further the fistula and for possible intervention. Coronary angiogram performed prior to surgery showed large RCA to RV fistula with two large openings into RV each measuring 6 mm in diameter. (Fig. 3)
Since there were two large exit points into the RV, she needed two 10 or 12 mm Amplatzer vascular plugs or two 8/6 Amplatzer PDA device occluders. Also, due to the small weight of the baby (3 kg), a large sheath would be needed for percutaneous procedure with related complications and hence she was advised surgery. She underwent urgent surgical ligation of the fistula along with atrial septal defect closure with uncomplicated recovery.
Figures 2A & 2B: (A) Transthoracic echocardiography showing connection of right coronary fistula into right ventricle. (B) Color Doppler shows turbulent blood flow communicating between the right coronary artery and right ventricle.
LV=Left ventricle. RV=Right ventricle.
Figure 3: Ascending aortogram showing a large dilated right coronary artery with two fistulous exit openings (arrow heads) into the right ventricle.
DISCUSSION
Coronary artery fistulas (CAF) are uncommon anomalies in which a communication exists between a coronary artery and a cardiac chamber (coronary-cameral fistula) or a great vessel (coronary arterio-venous fistula), bypassing the myocardial capillary network. They are mostly congenital but acquired forms can also occur due to trauma or iatrogenic cause (after cardiac surgery or transcatheter interventions).
Congenital CAF arise due to incomplete closure of embryonic intertrabecular spaces and coronary sinusoids. The functional disturbance caused by CAF is due to myocardial stealing secondary to a diastolic pressure gradient and runoff from high-pressure coronary artery to a low-pressure cardiac chamber with decrease in intracoronary diastolic perfusion pressure.
The coronary artery compensates by enlargement of the donor artery and may become aneurysmal with risk of thromboembolism and rarely, endocarditis and rupture.1
The hemodynamic consequences of CAF depend on their origin, size of the communication, the resistance of the recipient chamber, and the potential for development of myocardial ischemia and significant left-to-right shunt. The reported incidence of CAF is about 0.1-0.2% among all cardiac catheterizations.2 Approximately 50% of pediatric coronary anomalies are CAF. Concomitant congenital anomalies occur in 40% of patients. The origin of the fistulae is variable; right coronary artery (50%), left coronary artery (42%), both coronary arteries (5%). More than 90% of CAF drain into right heart; right ventricle (41%), right atrium (26%), pulmonary artery (17%) and the rest in coronary sinus, left atrium, left ventricle or superior vena cava.1
CAF are usually asymptomatic, especially in those less than 20 years of age and who have small CAF.3,4 They are accidentally discovered during echocardiography or coronary angiography. It tends to manifest in infants less than 2 years of age with heart failure; in young adults with angina, dyspnea on exertion, myocardial ischemia/infarction; and in adults more than 40 years of age with heart failure, atherosclerosis and arrhythmias.4 The presence of a continuous murmur in the lower sternal border is highly suggestive of a CAF. Differential diagnosis include PDA, ruptured sinus of Valsalva aneurysm, aortopulmonary window, supracristal ventricular septal defect with aortic regurgitation, internal mammary artery to pulmonary artery fistula, and pulmonary or systemic arteriovenous fistula.1,5
Traditionally, coronary angiography is the gold standard for imaging the coronary tree and also CAF. Noninvasive imaging with echocardiography, computed tomography, and magnetic resonance imaging (MRI) may facilitate the diagnosis of CAF.6 Transthoracic echocardiography with color Doppler is non-invasive, easily available, and fairly accurate in diagnosing many forms of CAF. Associated cardiac lesions and ventricular function can be evaluated, but the major limitation is that branching coronary vessels from the fistula are not recognizable. Limitation of transthoracic echocardiography was noted in our patient; namely only one exit orifice of the CAF was seen, and the other orifice was diagnosed by aortography. MRI is a good alternative for imaging proximal coronary anomalies, but not for distal course of CAF.
Multi-detector row computed tomography cardiac imaging has provided excellent distal coronary artery and side branch imaging with better temporal and spatial resolution than MRI and is considered by many as diagnostic modality of choice in imaging of coronary anomalies. If a decision is taken to perform percutaneous
embolization of the fistula, then cardiac catheterization is indicated.5
Small fistulas which are haemodynamically and clinically silent usually do not require any treatment. Patients with large fistula, symptoms of heart failure or myocardial ischemia and pediatric patients with high Qp/Qs should be treated early.
Treatment options include surgical repair (patch/suture closure or ligation),7,8 or transcatheter embolization.7,9,10 Mavroudis et al. in their series reported 100% success rate and survival with surgery and they recommend elective coil occlusion in patients who fulfill the following criteria; absence of multiple fistulas, a single narrow drainage site, absence of large branch vessels, and safe accessibility to the coronary artery supplying the fistula.7 Different materials are used in the embolization of CAF; releasable balloons, microcoils/hydrocoils, micro particles and Amplatzer occluder or plug. In asymptomatic cases, regular follow up is advised because there is a chance of spontaneous closure of small clinically silent fistulas.3,4
CONCLUSION
CAF are a rare cause of heart failure in early infancy. Detection of a continuous murmur in a site atypical for PDA and no clear-cut explanation during routine echocardiographic examination should raise the suspicion of CAF.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
-
Cutler JA, Thom TJ, Roccella E. Leading causes of death in the United States. JAMA 2006; 295:383-384.
-
Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc 1999; 58:163-169.
-
Levine GN, Keaney JF Jr, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med 1995; 332: 512-521.
-
The Scandinavian Simvastatin Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet 1994; 344: 1383-1389.
-
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1994; 333:1301-1307.
-
Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, et al. Linoleic Acid, Other Fatty Acids, and the Risk of Stroke. Stroke 2002; 33:2086-2093.
-
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989; 79:8-15.
-
Klimov AN, Gurevich VS, Nikiforova AA, Shatilina LV, Kuzmin AA, Plavinsky SL. Antioxidative activity of high density lipoproteins in vivo. Atherosclerosis 1993; 100:13-18.
-
Von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care 2005; 8:147-152.
-
Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc 1999; 58:163-169.
-
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986; 256:2835-2838.
-
Franceschini G. Epidemiologic evidence for high-density lipoprotein cholesterol as a risk factor for coronary artery disease. Am J Cardiol 2001; 88:9-13.
-
Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High-Density Lipoprotein Intervention Trial. Am J Cardiol 2000; 86:19-22.
-
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987; 317:1237-1245.
-
Tall AR. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest 1990; 86:379-384.
-
Albrink MJ, Man EB. Serum triglycerides in coronary artery disease. AMA Arch Intern Med 1959; 103:4-8.
-
Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996; 3:213-219.
-
Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 1998; 97:1029-1036.
-
Austin M. Plasma triglyceride and coronary heart disease. Arterioscler Thromb 1991; 11:2-14.
-
Krauss RM. Triglycerides and atherogenic lipoproteins: rationale for lipid management. Am J Med 1998; 105:58-62.
-
Koren E, Corder C, Mueller G, Centurion H, Hallum G, Fesmire J, et al. Triglyceride enriched lipoprotein particles correlate with the severity of coronary artery disease. Atherosclerosis 1996; 122:105-115.
-
Austin MA. Plasma triglyceride as a risk factor for cardiovascular disease. Can J Cardiol 1998; 14:14-17.
-
Oberman A. Hypertriglyceridemia and Coronary Heart Disease. J Clin Endocrinol Metab 2000; 85:2098-2105.
-
Asia Pacific Cohort Studies Collaboration. Serum Triglycerides as a Risk Factor for Cardiovascular Diseases in the Asia-Pacific Region. Circulation 2004; 110:2678-2686.
-
Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. PROVE IT-TIMI 22 Investigators. Impact of Triglyceride Levels Beyond Low-Density Lipoprotein Cholesterol After Acute Coronary Syndrome in the PROVE IT-TIMI 22 TrialJ Am Coll Cardiol 2008; 51:724-730.
-
Patsch JR, Miesenböck G, Hopferwieser T, Muhberger V, Knapp E, Dunn JK. Relation of triglyceride metabolism and coronary artery disease. Studies in the post-prandial state. Arterioscler Thromb 1992; 12:1336-1345.
-
Miesenböck G, Patsch JR. Postprandial hyperlipidemia: the search for the atherogenic lipoprotein. Curr Opin Lipidol 1992; 3:196-201.
-
Durrington PN. Triglycerides are more important in atherosclerosis than epidemiology has suggested. Atherosclerosis 1998; 141:57-62.
-
Williams CM. Postprandial lipid metabolism: effects of dietary fatty acids. Proc Nutr Soc 1997; 56:679-692.
-
Patsch JR, Prasad S, Gotto AM Jr, Patsch W. High density lipoprotein2. Relationship of the plasma levels of this lipoprotein species to its composition, to the magnitude of postprandial lipemia, and to the activities of lipoprotein lipase and hepatic lipase. J Clin Invest 1987; 80:341-347.
-
Schrezenmeir J, Weber P, Probst R, Biesalski HK, Luley C, Prellwitz W, et al. Postprandial pattern of triglyceride-rich lipoprotein in normal-weight humans after an oral lipid load: exaggerated triglycerides and altered insulin response in some subjects. Ann Nutr Metab 1992; 36:186-196.
-
Tall A, Sammett D, Granot E. Mechanisms of enhanced cholesteryl ester transfer from high density lipoproteins to apolipoprotein B-containing lipoproteins during alimentary lipemia. J Clin Invest 1986; 77:1163-1172.
-
Stensvold I, Tverdal A, Urdal P, Graff-Iversen S. Non-fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged Norwegian women. BMJ 1993; 307:1318-1322.
-
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 1996; 276:882-888.
-
Havel RJ. Postprandial hyperlipidemia and remnant lipoproteins. Curr Opin Lipidol 1994; 5:102-109.
-
Cohn JS. Postprandial lipid metabolism. Curr Opin Lipidol 1994; 5:185-190.
-
Alipour A, Elte JW, Van Zaanen HC, Rietveld AP, Castro Cabezas M. Novel aspects of postprandial lipemia in relation to atherosclerosis. Atheroscler Suppl 2008; 9:39-44.
-
Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1996; 93:1107-1113.
-
Ross R. The pathogenesis of atherosclerosis: a perspective for 1990s. Nature 1993; 326:801-809.
-
Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol 1997; 30:325-333.
-
Petrie JR, Ueda S, Morris AD, Murray LS, Elliot HL, Connell JM. How reproducible is bilateral plethysmography? Br J Clin Pharmacol 1998; 45:131-139.
-
Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997; 79:350-354.
-
Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, Tornvall P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulationm 1997; 96:3266-3268.
-
Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide production. J Clin Invest 1993; 91:2546-2551.
-
Shiode N, Kato M, Hiraoka A, Yamagata T, Matsuura H, Kajiyama G. Impaired endothelium-dependent vasodilation of coronary resistance vessels in hypercholesterolemic patients. Intern Med 1996; 35:89-93.
-
Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 1953; 265:1053-1057; contd
-
Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 1953; 265:1111-1120; concl.
-
Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health 1987; 8:253-287.
-
Berlin JA, Colditz GA. meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol 1990; 132:612-628.
-
Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung Dl, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993; 328:538-545.
-
Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 1993; 328:533-537.
-
Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of Leisure-Time Physical Activity and Cardiorespiratory Fitness to the Risk of Acute Myocardial Infarction in Men. N Engl J Med 1994; 330:1549-1554.
-
Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet 1998 351:1603-1608.
-
Fletcher GF, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, et al. Statement on exercise. Benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart association. Circulation 1992; 86:340-344.
-
Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW 3rd, Blair SN.
Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA 1999; 281:327-334. -
Whaley MH, Blair SN. Epidemiology of physical activity, physical fitness and coronary heart disease. J Cardiovasc Risk 1995; 2:289-295.
-
Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all cause mortality and longevity of college alumni. N Engl J Med 1986; 314:605-613.
-
Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991; 338:774-778.
-
Paffenbarger RS Jr, Hyde RT, Wing AL, Lee Im, Jung DL, Kampert B. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993; 328:538-545.
-
Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet 1998; 351:1603-1608.
-
Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med 1998; 338:94-99.
-
Schroeder ET, Hawkins SA, Hyslop D, Vallejo AF, Jensky NE, Wiswell RA. Longitudinal Change in Coronary Heart Disease Risk Factors in Older Runners. Age Ageing 2007; 36:57-62.
-
Oldridge N, Gottlieb M, Guyatt G, Jones N, Streiner D, Feeny D. Predictors of health-related quality of life with cardiac rehabilitation after acute myocardial infarction. J Cardiopulm Rehabil 1998; 18:95-103.
-
Lemaitre RN, Heckbert SR, Psaty BM, Siscovick DS. Leisure-time physical activity and the risk of nonfatal myocardial infarction in postmenopausal women. Arch Intern Med 1995; 155:2302-2308.
-
Wannamethee SG, Shaper AG, Walker M. Physical activity and mortality in older men with diagnosed coronary heart disease. Circulation 2000; 102:1358-1363.
-
Hsieh SD, Yoshinaga H, Muto T, Sakurai Y. Regular physical activity and coronary risk factors in Japanese men. Circulation 1998; 97:661-615.
-
Williams PT. Lipoproteins and adiposity show improvement at substantially higher exercise levels than those currently recommended. Circulation 1994; 90:I-471.
-
Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C. Physical activity and public health: a recommendation from the CDC and ACSM. JAMA 1995; 273:402-407.
-
Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary Prevention of Ischemic Stroke: A Guideline From the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:1583-1633.
-
Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev 1994; 22:477-521.
-
Durstine JL, Pate RR, Sparling PB, Wilson GE, Senn MD, Bartoli WP. Lipid, lipoprotein, and iron status of elite women distance runners. Int J Sports Med 1987; 8:119-123.
-
Kokkinos PF, Holland JC, Narayan P, Colleran JA, Dotson CO, Papademetriou V. Miles run per week and high-density lipoprotein cholesterol levels in healthy, middle-aged men. A dose-response relationship. Arch Intern Med 1995; 155: 415-420.
-
Miesenböck G, Patsch JR. Postprandial hyperlipidemia: the search for the atherogenic lipoprotein. Curr Opin Lipidol 1992; 3:196-201.
-
Gordon NF, Scott CB, Wilkinson WJ, Duncan JJ, Blair SN. Exercise and mild essential hypertension. Recommendations for adults. Sports Med 1990; 10:390-404.
-
Vranic M, Wasserman D. Exercise, fitness, and diabetes. In: Bouchard C, Shephard RJ, Stephens T, Sutton J, McPherson B, eds. Exercise, Fitness and Health: A Consensus of Current Knowledge. Champaign, Ill: Human Kinetics Books 1990 p.467-490.
-
Hughes RA, Thorland WG, Housh TJ, Johnson GO. The effect of exercise intensity on serum lipoprotein responses. J Sports Med Phy Fitness 1990; 30: 254-260.
-
Drygas W, Kostka T, Jegier A, Kunski H. Long-term effects of different physical activity levels on coronary heart disease risk factors in middle-aged men. Int J Sports Med 2000; 21:235-241.
-
Crouse SF, O’Brien BC, Grandjean PW, Lowe RC, Rohack JJ, Green SJ, et al. Training intensity, blood lipids, and apolipoproteins in men with high cholesterol. J Appl Physiol. 1997; 82:270-277.
-
Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, et al. Effect of Aerobic Exercise Training on Serum Levels of High-Density Lipoprotein Cholesterol: A Meta-analysis. Arch Intern Med 2007; 167:999-1008.
-
McAllister RM, Hirai T, Musch TI. Contribution of endothelium-derived nitric oxide (EDNO) to the skeletal muscle blood flow response to exercise. Med Sci Sports Exerc 1995; 27: 1145-51.
-
Green DJ, O‘Driscoll G, Blanksby BA, Taylor RR. Control of skeletal muscle blood flow during dynamic exercise: contribution of endothelium-derived nitric oxide. Sports Med 1996; 21: 119-46.
-
Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol 1996; 28: 1652-60
-
Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 1994; 74: 349-53.
-
Hambrecht R, Wolf A, Gielen S. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 2000; 42: 454-460.
-
Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et.al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998; 98: 2709-15.
-
Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 1996; 93: 210-4.
-
Delep MD, McAllister RM, Laughline MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol 1993; 75: 1354–1363.
-
Gordon NF, Scott CB, Wilkinson WJ, Duncan JJ, Blair SN. Exercise and mild essential hypertension. Recommendations for adults. Sports Med 1990; 10: 390-404.
-
Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation 1999; 100: 1194-202
-
Gordon NF, Scott CB, Wilkinson WJ, Duncan JJ, Blair SN. Exercise and mild essential hypertension. Recommendations for adults. Sports Med 1990; 10: 390-404.
-
Goto, C., Higashi, Y., Kimura, M., Noma, K., Hara, K., Nakagawa, et al. The effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 1993; 108: 530–535.
-
Jason M. R. Gill, Ali Al-Mamari, William R. Ferrell, Stephen J. Cleland, Chris J. Packard, Naveed Sattar, et al. Effects of Prior Moderate Exercise on Postprandial Metabolism and Vascular Function in Lean and Centrally Obese Men. (J Am Coll Cardiol 2004; 44:2375– 82
-
Gill JM, Al-Mamari A, Ferrell WR, Cleland SJ, Perry CG, Sattar N, et al. Effect of prior moderate exercise on postprandial metabolism in men with type 2 diabetes: heterogeneity of responses. Atherosclerosis 2007; 194:134-43.
How to cite this URL
Al-Maskari S, PAnduranga P, Al-Farqani A. Coronary Artery Fistula with Heart Failure in Early Infancy. OMJ [Online] 2010 July; 25(3). Available at http://www.omjournal.org/CaseReports/FullText/201007/FT_8CoronaryArteryFistulawithHeart.html.