The Rhesus (Rh) blood group system was discovered by Landsteiner and Wiener in 1940.1 Rh alloimmunization in pregnancy develops when the maternal red blood cells (RBCs) lacking the Rh antigen (RhD negative) are exposed to RhD positive RBCs through the placenta leading to the activation of the maternal immune system. This causes the production of antibodies against RhD positive blood cells, which is termed sensitization. Sensitization of mother’s blood not only occurs through the placenta, but due to blood transfusion, miscarriage, and ectopic pregnancy, and in procedures such as amniocentesis.1 Immunoglobulin G (IgG) antibodies to the Rh antigen cross the placenta and bind to fetal RhD positive RBCs, which leads to destruction of fetal RBCs, resulting in hemolytic anemia. It may also cause brain damage (due to high bilirubin levels as a result of RBC destruction), and even death due to hydrops fetalis.2 Before the
practice of passive administration of anti-D IgG, the risk of immunization was calculated as 13.2%.3 Postpartum immunoprophylaxis decreased the incidence of post-pregnancy anti-D immunization to 1–2%.4,5 A Canadian study showed that in 1,357 RhD-negative primigravida the incidence of RhD alloimmunization could be reduced to 0.1% by prophylaxis with antenatal anti-D IgG, in addition to postpartum prophylaxis.3 There is currently sufficient evidence demonstrating that antenatal anti-D prophylaxis also reduces the risk of RhD immunization in the next pregnancy to below the level of 0.4%.6 The recommendations for postpartum and antenatal administration of anti-D were described by Fung and Eason et al,7 and are currently followed internationally. In Oman, the procedure for RhD negative women includes one dose of anti-D immunization at 28 and 34 weeks, and one dose postnatally after checking the neonatal blood group and direct Coombs test. Despite this preventive measure, alloimmunization due to anti-D, anti-c, and anti-Kell are commonly seen as the most common causes of hemolytic disease of the fetus and newborn (HDFN).8
The prevalence of RhD negativity varies from race to race. Approximately 15% of Caucasians are RhD negative while the number is 5–8% of African Americans and 1–2% of Asians and Native Americans.9 Indigenous Africans are virtually 100% RhD positive.10
Oman is a developing country with a genetically admixed population consisting of Caucasian, African, and Asian ancestries and there are no studies on the prevalence of distribution of ABO blood group and Rh status. The aim of our study was to provide information about the prevalence of RhD negative status in pregnant women, the distribution of ABO blood groups, and the rate of RhD alloimmunization in pregnancy.
Methods
We conducted a retrospective review of hospital records of pregnant women who delivered at the Sultan Qaboos University Hospital (SQUH) between June 2011 and June 2013. All pregnant women were tested for ABO blood type, and RhD antigen and antibody screening at their first antenatal clinic visit. The study group included all Omani pregnant women who registered for delivery at the obstetrics and gynecology department in SQUH. Women registered elsewhere but who delivered in SQUH were excluded.
The study was approved by Sultan Qaboos University Ethical Review Committee and Obstetrics and Gynecology Department at Sultan Qaboos University (MREC no 811).
Data was collected from the Hospital Information System (HIS). Women who were negative for RhD antigen were tested for the presence of antibodies to estimate the rate of RhD alloimmunization. Women who had positive anti-D antibodies were further analyzed to determine if they had the active (true) RhD alloimmunization or passive (false) RhD alloimmunization. Antibody detection was performed by the gel card technique (BIORAD ID-System; DiaClon, Bio-Rad, DiaMed GmbH, Switzerland). The reliability of antigen detection is largely dependent on the availability of test cells with appropriate antigens and the sensitivity of the test methods. Test cells reagents used for antibody screening were BIORAD© ID-DiaCell I-II and ID-DiaCell I-II-III, and for antibody identification we used the ID-Panel, and ID-Panel-P (Bio-Rad, DiaMed GmbH, Switzerland). In women who tested positive for these antibodies (active/true) an antibody titer was performed to evaluate the severity of their case.
Data was collected on maternal characteristics including the area of residence, age, gravidity, parity, medical history, history of previous blood transfusion, and previous miscarriage.
Results
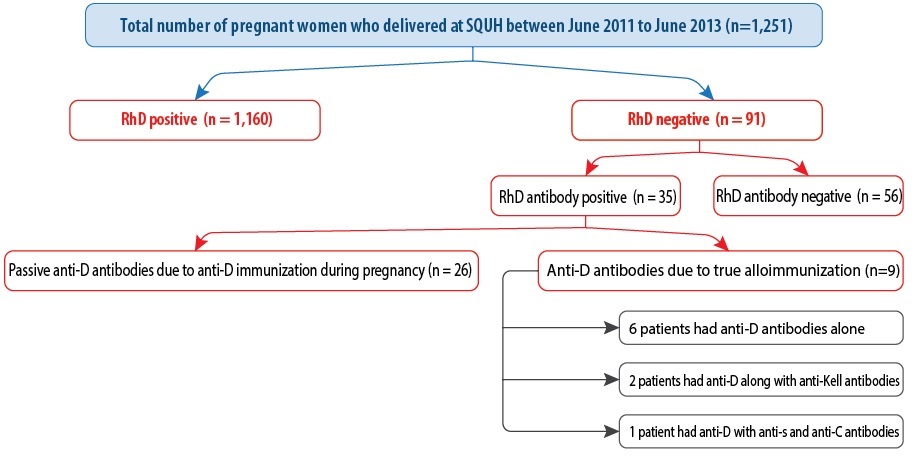
Figure 1: Flow chart describing the rate of rhesus alloimmunization.
A total of 1,251 pregnant women who were managed and delivered at SQUH were included in the study. Of these, 1,160 (92.7%) women were RhD positive and 91 (7.3%) were RhD negative. The most common blood type was O followed by A, B, and AB [Table 1]. True alloimmunization occurred in 10% (n = 9) of women. Four women had previous alloimmunization, and three women had a cesarean section in a past pregnancy. Four women had previously miscarried. Neonatal jaundice occurred in six babies, and the other complications included polyhydramnios (n = 3), respiratory distress syndrome (n = 2), and postnatal anemia (n = 1).
Table 1: The distribution of different blood groups and RhD factor among pregnant women at SQUH from June 2011 to June 2013 (n = 1,251).
|
O + |
615 |
49.2 |
|
O - |
38 |
3.0 |
|
Total |
653 |
52.2 |
|
A + |
299 |
23.9 |
|
A - |
32 |
2.6 |
|
Total |
331 |
26.5 |
|
B + |
185 |
14.8 |
|
B - |
19 |
1.5 |
|
Total |
204 |
16.3 |
|
AB + |
61 |
4.9 |
|
AB - |
2 |
0.2 |
Of the 91 (7.3%) RhD negative women, 35 (38.5%) patients had antibodies to the RhD antigen [Figure 1]. Among the 35 patients detected with positive antibodies, 26 had passive or false alloimmunization because of the effect of receiving a dose of RhD immunoglobulin (anti-D) as evident by a history of receiving an anti-D injection and a low titer (1:1 to 1:8). Only nine patients developed active or true alloimmunization. Therefore, the rate of RhD negative alloimmunization in pregnancy was 10% (9/91). Amongst the nine patients with positive antibodies, the RhD antibody pattern was as follows: six (66.7%) patients with only anti-D detected, two (22.2%) patients with anti-D combined with anti-Kell, and one (11.1%) patient of combined anti-D, anti-s and anti-C.
We reviewed the past medical history of the nine pregnant women with positive antibodies. The mean maternal age was 32 years (range = 23–44 years). Eight (88.9%) women were multiparous, and one (11.1%) woman was primigravida. The mean gravidity was four (range = 3–8), and the mean parity was one (range = 0–4). Four women (44.4%) had a previous miscarriage with an abortion frequency ranged between one and three times. None of the patients had a previous history of blood transfusion. The possible reasons behind alloimmunization are given in Figure 1. Some women had more than one parameter for positive antibodies.
There was no fetus with hydrops. The minimum and maximum anti-D titer were 1:1 and 1:8 in the passive immunization group, respectively. In the actively sensitized women, the minimum anti-D titer was 1:2 and the maximum was 1:256. The mean gestational age at delivery was 37 weeks ±3 days, and the mean neonatal hemoglobin was 16 g/dL. The mean birth weight of the neonates was 2.8 kg. Only one baby with hemoglobin levels of 12 g/dL needed a transfusion.
Discussion
Studying the prevalence of RhD negative women is an important step to minimize the complications of RhD alloimmunization in pregnancy. It will also aid the development of screening and preventive management programs based on the calculated prevalence. The prevalence of the RhD negative blood type among pregnant women in Oman was 7.3%. In a study from Saudi Arabia the prevalence was 7.5%,9 the same as another study from South India by Varghese et al.11 It is interesting to note that the prevalence of RhD negativity in the Omani population falls closer to the prevalence of 5–8% of African Americans. This is important as, historically, Oman had cultural and trade relations with East Africa and as such the current population in Oman is an admixture between Caucasian, African, and Asian ancestries.12
Only nine patients out of 91 developed true or active alloimmunization. Importantly, this rate was very high compared to the rate of 1.8% in Saudi Arabia.9 We compared the clinically significant alloimmunization among the RhD negative women to the study by Varghese et al,11 but the alloimmunization in RhD negative women was much higher in our study. Although there was no history of documented transfusion, our results suggest that some women in the group who had immunization from previous pregnancies received a blood transfusion for hemoglobinopathy or postpartum hemorrhage. This is quite likely, as the presence of underlying significant hemoglobinopathy in the Omani population is about 10%.13 A previous history of miscarriage was positive in four of the nine women who were sensitized.
Although this high prevalence was due to alloimmunization in pregnancies (miscarriage/delivery), only one fetus developed anemia and needed a postnatal transfusion. Furthermore, there was no fetus with hydrops. Prevention programs in the form of antenatal and postnatal immunization are already in place in Oman. These should be further supported and strengthened by organized nationwide neonatal screening programs, and utilization of anti-D immunoglobulin at the appropriate time and dosages according to the currently recommended international protocols.
There are inherent limitations due to the retrospective nature of the study. Information on blood transfusion in previous pregnancies and during miscarriages, and possibility of misclassification of the Rh typing (rhesus negative classified as positive) in the interior hospitals might be some of the reasons for the high prevalence of alloimmunization.
Conclusion
Our study found the prevalence of RhD negativity in pregnant Omani women (7.3%) was comparable to other Asian countries. The rate of RhD negative alloimmunization in the present study was 10%. Previous RhD alloimmunization during pregnancy and history of miscarriage were the most common maternal medical history.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Landsteiner K, Wiener AS. An agglutinable factor in human blood recognized by immune sera for rhesus blood. Proc Soc Exp Biol 1940;43: 223.
- 2. Avent ND, Reid ME. The Rh blood group system: a review. Blood 2000 Jan;95(2):375–387.
- 3. Bowman J. Rh-immunoglobulin: Rh prophylaxis. Best Pract Res Clin Haematol 2006;19(1):27–34.
- 4. Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al; British Society for Haematology. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014 Feb;24(1):8–20.
- 5. Hartwell EA; American Society of Clinical Pathologists. Use of Rh immune globulin: ASCP practice parameter. Am J Clin Pathol 1998 Sep;110(3):281–292.
- 6. Liumbruno GM, D’Alessandro A, Rea F, Piccinini V, Catalano L, Calizzani G, et al. The role of antenatal immunoprophylaxis in the prevention of maternal-foetal anti-Rh(D) alloimmunisation. Blood Transfus 2010 Jan;8(1):8–16.
- 7. Fung Kee Fung K, Eason E, Crane J, Armson A, De La Ronde S, Farine D, et al; Maternal-Fetal Medicine Committee, Genetics Committee. Prevention of Rh alloimmunization. J Obstet Gynaecol Can 2003 Sep;25(9):765–773.
- 8. Moise KJ Jr. Non-anti-D antibodies in red-cell alloimmunization. Eur J Obstet Gynecol Reprod Biol 2000 Sep;92(1):75–81.
- 9. Bondagji NS. Rhesus alloimmunization in pregnancy. A tertiary care center experience in the Western region of Saudi Arabia. Saudi Med J 2011 Oct;32(10):1039–1045.
- 10. Sarhan MA, Saleh KA, Bin-Dajem SM. Distribution of ABO blood groups and rhesus factor in Southwest Saudi Arabia. Saudi Med J 2009 Jan;30(1):116–119.
- 11. Varghese J, Chacko MP, Rajaiah M, Daniel D. Red cell alloimmunization among antenatal women attending a tertiary care hospital in south India. Indian J Med Res 2013;138:68–71.
- 12. Pathare AV, Al Zadjali S, Misquith R, Alkindi SS, Panjwani V, Lapoumeroulie C, et al. Warfarin pharmacogenetics: polymorphisms of the CYP2C9, CYP4F2, and VKORC1 loci in a genetically admixed Omani population. Hum Biol 2012 Feb;84(1):67–77.
- 13. Alkindi S, Al Zadjali S, Al Madhani A, Daar S, Al Haddabi H, Al Abri Q, et al. Forecasting hemoglobinopathy burden through neonatal screening in Omani neonates. Hemoglobin 2010 Jan;34(2):135–144.