| |
How to cite this Article
Wani I, Bhat IHG, Mir M, Mir M, Hassan N, Mustafa A. Pyoderma Gangrenosum of Abdominal Wall. OMJ 2011 Jan; 26(1): 64-65.
How to cite this URL
Wani I, Bhat IHG, Mir M, Mir M, Hassan N, Mustafa A. Pyoderma Gangrenosum of Abdominal Wall. OMJ 2011 Jan; 26(1): 64-66. Available from http://www.omjournal.org/fultext_PDF.aspx?DetailsID=68&type=fultext
|
|
| |
Pyoderma gangrenosum, a nuetrphilic dermatosis, is a rare ulcerative cutaneous disease. An immunological abnormality has been proposed as a possible mechanism. There are no specific clinical features seen with this disease. This has a characteristic feature of pathergy. Diagnoses rely on excluding other causes of ulcerative skin diseases. The occurrence of pyoderma gangrenosum at a surgical site is rare especially if there is no predisposing illness. Early diagnosis is essential to prevent pathergy. Corticosteroids are main drugs used for treatment.
A 28 year old female presented with painful swelling of right abdominal wall and fever of 5 days duration for which she had received antibiotics but had no relief, with the worsening of symptoms and progressive increase of swelling. On general physical examination, patient had dehydrated look and fever of 100 degrees Fahrenheit. Systemic examination was normal. Local examination revealed a tender swelling measuring 10 ×7.8 × 2.3 centimeter showing fluctuation, free from underlying structures, with prominence on Carnett’s test’s (leg raising test) in right lower abdomen and was diagnosed as right parietal wall abscess, and had an incision drainage. Thirty six hours after incision drainage, despite of improvement, there was seen ulcer with pus and development of another pustule, and patient underwent another debridement. There was seen further worsening of symptoms Despite patient being on broad spectrum antibiotics and drainage of abscess there was no improvement and patient developed third ulcerated lesion adjacent to previous ones, the ulcers having dirty base with asymmetrical borders (Fig.1). Histopathological examination identified a non-specific mixed inflammatory cell infiltrate with areas of necrosis. Cultures of debrided tissue were negative for bacteria, mycobacteria, and fungi. Serum protein electrophoresis was normal, antiphospholipid, antinuclear and antineutrophil cytoplasmic antibodies and rheumatoid factor was negative. No associated disease was identified. Patient was diagnosed as a case of pyoderma gangrenosum and was put on corticosteroid and had well response and had the lesion healed. No fresh recurrence had seen in follow up after follow up of 9 months.
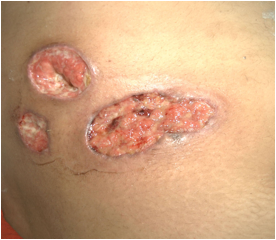
Figure 1: Ulcers having dirty base with asymmetrical borders (Pyoderma Gangrenosum)
Pyoderma gangrenosum was first reported in 1924 following drainage of an abdominal abscess and is regarded as a diagnosis of exclusion.1 It is categorized into four types; ulcerated pyoderma, bullous, pustular and vegetans, each with its own peculiarities and different clinical responses to treatment. The ulcerative form of pyoderma gangrenosum is characterized by a rapidly progressing painful irregular and undermined bordered necrotic ulcer.2
Lesions can be solitary or multiple, chronic or recurrent.Any site on body is prone for developing this skin dermatosis. They commonly occur on legs, especially on pretibial area, face, neck, scrotum, or penis. Pyoderma gangrenosum of abdominal wall adjacent to stoma following colectomy can occur. Post-surgical pyoderma gangrenosum can affect any surgical wound. However, the abdominal wall and the breasts are the most affected areas.3
About 50% of the cases of pyoderma gangrenosum occur concomitantly with other diseases, notably with systemic diseases especially arthritides, inflammatory bowel disease and hematological disorders (leukemia) disorder, with inflammatory bowel disease being the most common assocaition. Less common associated diseases include hepatic diseases, myelomas, immunological diseases and HIV infection.4 The remaining forms occur in an isolated manner. Lesions can develop spontaneously, after surgery or trauma. An immunological anomaly of the hyperergic reaction type, involving IL-8 (interleukin-8) and IL-16 (interleukin-16) have been implicated in etiology of pyoderma gangrenosum. Mixed genetic predisposition, undefined infectious agents or paraneoplastic or paraimmune phenomenon are the factors maintaining these abnormalities.5
Pyoderma ulcers are classically painful and aseptic, although super infection may eventually occur. Sequence of events occurs in the formation of ulcerative lesion; initially pustule or inflammatory nodule appears which breaks down to form an ulcer. This ulcer shows purulent base with ragged undermined assymetrical border sometimes bluish edge which spreads centrifugally. The distinctive clinical features of pyoderma gangrenosum are apparent enough to permit the diagnosis of most cases, comprising in its classic form a burrowing ulcer with an irregular margin and ragged purple-red overhanging edge. The extension of lesions of pyoderma gangrenosum in response to trauma or surgical debridement, termed pathergy, is a hallmark of this entity, is observed in 25–50% of cases.6 Sometimes in presence of multiple ulcers of pyoderma gangrenosum, they may colesce resulting in large ulcer which usually heal with pigmented cribriform scar. Histopathological examination of ulcer varies with stage of disease. In early case, nuetrophilic abscess is seen followed by epidermal necrosis and ulceration, superficial dermal edema and a dense mixed dermal infiltration in later stages whereas biopsy of advancing inflamed border demonstrates perivascular lymphatic inflammation.
The rarity of the disease, the condition's ability to mimic other ulcerative lesions and its lack of specific laboratory and pathologic findings leads to misdiagnosis and mistreatment of the lesion. A high index of clinical suspicion can lead to a definitive diagnosis. The frequency of misdiagnosing is about 10%. Its treatment is still a motive for discussion in the literature with no uniformly effective or specific therapy for pyoderma gangrenosum. Immunosuppression is the basis of treatment. Debridement or necrosectomy in postoperative pyoderma gangrenosum is contraindicated.7
Corticosteroids and cyclosporine are the most commonly used drugs as immunosuppresants. Topical treatment includes topical or intralesional corticosteroids, tacrolimus ointment, intralesional cyclosporine, topical 5-aminosalicylic acid, nitrogen mustard or 0,5% nicotine cream.8 These topical agents are generally used as supportive treatment for systemic treatment and their use as monotherapy has a limited role. In cases without associated disease, intravenous immunoglobulins, granulocyte and monocyte adsorption apheresis plasmapheresis and cyclophosphamide treatment are have been reported to be effective.
Pyoderma gangrenosum of abdominal wall is a rare disease. It can be easily misdiagnosed with other ulcerative skin diseases and pathergy is important for diagnosis.
Acknowledgements
The authors reported no conflict of interest and no funding has been received on this work.
|
|
| |
- Cullen TS. A progressively enlarging ulcer of the abdominal wall involving skin and fat, following drainage of an abdominal abscess apparently from appendiceal origin. Surg Gynecol Obstet 1924;38:579-582.
- Inan I, Myers PO, Braun R, Hagen ME, Morel P. Pyoderma gangrenosum after totally implanted central venous access device insertion. World J Surg Oncol 2008;6:31.
- Ouazzani A, Berthe J-V, de Fontaine S. Post-surgical pyoderma gangrenosum: a clinical entity. Acta Chir Belg 2007 Jul-Aug;107(4):424-428.
- Banga F, Schuitemaker N, Meijer P. Pyoderma gangrenosum after caesarean section: a case report. Reprod Health 2006;3:9.
- Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004 Nov;43(11):790-800.
- Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr, White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore) 2000 Jan;79(1):37-46.
- Ehling A, Karrer S, Klebl F, Schäffler A, Müller-Ladner U. Therapeutic management of pyoderma gangrenosum. Arthritis Rheum 2004 Oct;50(10):3076-3084.
- Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005 Aug;53(2):273-283.
|
|