The prevalence of allergic rhinitis (AR) is increasing globally. There is a paucity of information regarding the prevalence of AR and related comorbidities among Omani adults. The Sultanate of Oman occupies the south-eastern corner of the Arabian Peninsula and has a variety of topographical features consisting of coastal plains, mountains, valleys, and deserts. The climate is hot and humid in coastal areas, and hot and dry in the interior. A study to determine the prevalence of allergies in Omani school children was found to range from 7.5–20% in different age groups.1
Although the risk factors for AR are well identified, the role of indoor and outdoor pollution has yet to be understood both for the occurrence of the disease, and its manifestations.2 Identifying the most relevant allergens in each environment is also important.3 AR, non-allergic rhinitis (NAR), and infectious rhinitis have nasal hyper-reactivity to various stimuli.4 Otolaryngology literature describing management outcomes in patients with allergic rhinitis is also lacking.5
The primary objective of this study was to estimate the prevalence of AR and associated co-morbidities in Oman. The secondary objective was to identify knowledge gaps that need to be addressed in future AR research.
AR is an immunoglobulin E (IgE) mediated inflammation of the nasal membranes induced by allergen exposure, and is characterized by sneezing, nasal congestion, nasal itching, or rhinorrhea and may be associated with watering, redness of eyes, anosmia, and fatigue.6 Clinically, nasal features of AR can include nasal crease (allergic salute), which is a horizontal crease across the lower half of the nasal bridge (caused by repeated upward rubbing of the tip of the nose by the palm of the hand), and thin, watery nasal secretions. The mucosa of the nasal turbinates may be swollen, have a pale, bluish-grey color, or predominant erythema. Other features of AR may include congestion and swelling of the palpebral conjunctivae with excess tear production, dark circles around the eyes (“allergic shiners”), retraction of the tympanic membrane, prominent tissue on the posterior pharynx (cobble stoning), tonsillar hypertrophy, and a high-arched palate. Complications of AR include: sinusitis, otitis media, sleep disturbances (including apnea), dental problems (overbite) due to excessive mouth breathing, and Eustachian tube dysfunction.7
The causes of AR may differ depending on whether the symptoms are seasonal, perennial, or sporadic/episodic. Some patients are sensitive to multiple allergens and can have perennial AR with seasonal exacerbations. Allergy to seasonal pollens (tree, grass, and weed) and outdoor molds (Alternaria or Aspergillus, which are less often indoors) is the most common cause of seasonal AR. Perennial AR is typically caused by allergens within the home like dust mite species Dermatophagoides farinae and Dermatophagoides pteronyssinus, pets, such as cats and dogs, and cockroaches in infested households.8
The most commonly used methods of determining allergy to a particular substance are allergic skin testing and radioallergosorbent test (RAST). Allergy skin testing, an in vivo test for immediate IgE hypersensitivity reaction, is done by introducing an extract of a suspected allergen percutaneously. The antigen in the extract binds to IgE on skin mast cells, leading to the early-phase (immediate-type) reaction, release of mediators such as histamine, within 15–20 minutes causing the wheal-and-flare reaction (central wheal by infiltrating fluid, and surrounding erythema due to vasodilation, with concomitant itching). The size of reaction correlates with the degree of sensitivity to the allergen. RAST, an in vitro allergic test, measures the amount of specific IgE to individual allergens in a sample of patient’s blood. However, the sensitivity and specificity of RAST is not always as good as skin testing. Total serum IgE and total blood eosinophil count are neither sensitive nor specific for AR, but the results may be helpful in some patients when combined with other factors.9
Based on Allergic Rhinitis and its Impact on Asthma (ARIA) 2010 revision guidelines,10,11 reducing allergen exposure has been recommended in all patients with allergy to dust mite, indoor mould, animal dander, and occupational allergens. Intranasal glucocorticosteroids is recommended for the treatment of AR (seasonal/perennial) in adults and children not less than two-years-old.10-12 New generation oral H1-antihistamines is also recommended in adults with seasonal, perennial, or persistent AR.10,11,13
The use of oral leukotriene receptor antagonists (monteleukast) is not indicated for adults with perennial AR with doubtful recommendation (as low quality evidence) in adults and children with seasonal AR and in preschool children with perennial AR.10,11,14 Oral H1-antihistamine use is suggested over oral leukotriene receptor antagonists, taking into consideration the expense and availability of the drug. Research suggests that intranasal ipratropium bromide is beneficial only in patients experiencing AR with rhinorrhea. A short course of oral glucocorticosteroids is recommended only in patients with moderate to severe nasal and/or ocular symptoms that are not controlled with other treatments. Oral glucocorticosteroids should be avoided in children and pregnant women. Evidence shows that oral decongestants should not be administered alone or in combination with oral H1-antihistamine in AR.10 Sublingual allergen specific immunotherapy (SLIT) is recommended in both adults and children with AR due to pollen, and in adults only with AR due to house dust mites.15 Subcutaneous allergen specific immunotherapy (SCIT) is recommended in adults with seasonal/perennial AR due to house dust mites and in children with AR, although the evidence for the latter is not strong.10,11 A 2012 meta-analysis looking at the efficacy of SLIT and SCIT in patients with seasonal allergic rhinoconjuntivitis with grass allergens reported that SCIT is more effective than SLIT in controlling symptoms and in reducing the use of allergy medications.15
The relevance of AR should not be underestimated, as patients with AR and associated conditions, such as chronic rhinosinusitis, otitis media, and bronchial asthma, constitute a large population of patients attending day-to-day practices of otolaryngologists. Perennial allergic inflammation often presents as chronic inflammatory rhinitis without acute allergic symptoms. Recently a population-based, case-control study also found a strong association with atopic diseases (AD), including AR and attention-deficit hyperactivity disorder (ADHD), in children.16,17
Methods
This was a prospective cross sectional descriptive study of all consecutive patients presenting to the otolaryngology clinic of Sultan Qaboos University Hospital (SQUH), a tertiary care center in Oman between June 2010 and June 2011. The study was approved by college of medicine and health sciences Ethics committee.
Omani patients, male and female, aged between 18 and 70 years old with duration of symptoms of one year or more were included in the study. Patients with symptoms suggestive of chronic rhinosinusitis,18 and laryngopharyngeal reflux were excluded [Figure 1].
The diagnosis of AR was based on a thorough clinical history and confirmed by skin prick test,2,9 the clinical indicators being nasal symptoms of obstruction, rhinorrhoea, sneezing, and postnasal drip. Any history of anosmia, sleep disturbance, fatigue, pruritus, and ocular symptoms in particular were recorded. An association with exposure to known allergens was also recorded in addition to identifiable aeroallergen triggers, presence of concurrent allergic conditions, family history of AR, and respiratory irritants like smoking shisha, are inducers of exacerbation.19 The subjective severity was recorded; each symptom was evaluated on the following scale: 0 = absent, 1 = mild (symptom was present but not annoying or troublesome), 2 = moderate (symptom was frequently troublesome but did not interfere with normal activity or sleep), and 3 = severe (symptom interfered with activities and sleep).2 Based on the duration of the symptoms, patients were classified as seasonal AR and perennial AR. In addition, all patients had nasal endoscopy, skin prick tests and computed tomography (CT) sinuses as part of the diagnostic protocol [Figure 1].
The ‘gold standard’ for allergy testing is generally considered to be skin testing.9,20 Patients with positive skin prick tests were diagnosed as AR. A subset of patients who had positive CT findings for osteomeatal block, sinus opacities, nasal polyps in addition to positive skin tests were diagnosed as AR with chronic rhinosinusitis.
Descriptive and comparative analyses obtained using Statistical Package for the Social Sciences (SPSS) version 13.0. The demographic data and clinical profile data were expressed in percentages.
Results
A total of 887 patients were seen in otolaryngology clinics with nasal complaints; the mean age of presentation was 27 years. Non-infective rhinitis was diagnosed in 127 patients. Among them, 61 patients were noted to have AR (48%, 95% CI 39.3–56.7) compared to 66 patients with non-allergic rhinitis (52%, 95% CI 39.3–56.7). The prevalence of AR in the clinic was 7% (36 females with 25 males) and aged from 18–51 years. Fifty-one patients had perennial AR and 10 patients had seasonal AR. The prevalence of perennial AR was 84% (95% CI 74.8–93.2) compared to seasonal AR which was 16% (95% CI 6.9–25.2).
The most common perennial antigens were house dust mites, noted in 49 patients (80%, 95% CI 70–90), and cockroaches, in 41 patients (67%, 95% CI 55.2–78.8). Many patients had sensitivity to one or more antigens. The most common seasonal antigen was Russian thistle. All 10 patients diagnosed with seasonal AR were sensitive to this antigen.
Twenty-one patients were noted to have associated chronic rhinosinusitis, based on symptoms, endoscopic nasal examination, and CT scan of the sinuses, the prevalence being 34% (95% CI 22.5–46.3)
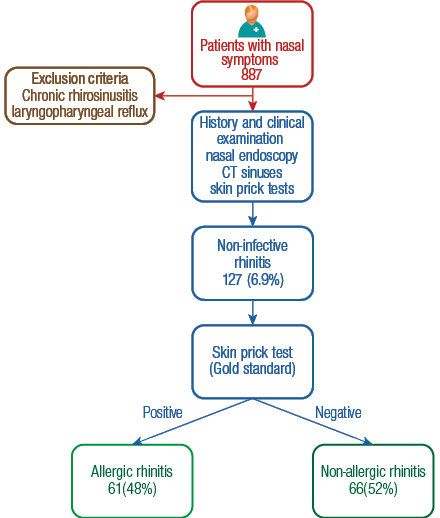
Figure 1: Diagnosis and study inclusion algorithm for prospective, cross-sectional study to estimate the prevalence of allergic rhinitis and associated co-morbidities among adults in Oman.
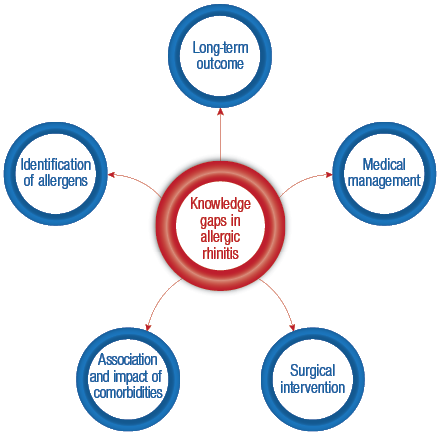
Figure 2: Knowledge gaps identified in allergic rhinitis research.
Discussion
Prevalence figures for AR vary widely from 0.8–40%.19,20 In our study, the prevalence for specialist tertiary care setting was found to be 7%, which compared to the prevalence of NAR given in the literature is lower.4 We found the prevalence of perennial AR was much higher (84%) compared to seasonal AR (16%). One reason could be the geographical and topographical features leading to lack of vegetation and pollen in Oman’s desert terrain, the same factors explain the low prevalence of seasonal AR. The most common perennial antigens noted were house dust mites (80%), followed by cockroaches (67%). The perennial allergens are those that might be present regardless of season. These include moulds, dust mites, and animal dander. Dust mites thrive on dead skin and warm (65oF to 80oF) and humid (50–70% relative humidity) conditions, like Oman’s climate.1,19
Seasonal allergens are usually pollen, as a general rule the sequence proceeds from grasses in the spring to trees in the summer.8 In Oman, most of the vegetation cultivated Russian thistle, a tree that pollinates during spring. This was the most common offender (100%) for the small subset of patients having seasonal AR in this study. The prevalence of chronic rhinosinusitis has not been previously reported. The prevalence was 34% for patients with AR in our study.
The accepted theory of the natural history of rhinitis and asthma is that the allergy starts in childhood with atopic dermatitis, progressing to asthma and then to AR. However, many patients develop rhinitis as their first symptom. Better epidemiological evidence is needed to understand the genetic background and the nature of this progression and whether prevention is possible.20 In the present study, the age of presentation ranged from 15 to 35 years.
AR may present as a distinct entity, coexist, or contribute to other states such as chronic rhinosinusitis, bronchial asthma, and laryngitis. Although there is some literature on association and impact of AR on bronchial asthma, this study could not address that issue. In addition, it was not possible to identify AR influencing on otitis media, or persistent pharyngitis/laryngitis. The role of food allergy was also not assessed. The limitations of our present study suggest the need for further prospective research.
There is also a paucity of outcome research with reference to the treatment of AR with many of the currently recommended guidelines because of the low quality evidence available.10 These gaps in the literature suggest the possible direction of future research looking at AR in Oman [Figure 2].
Conclusion
The prevalence of AR in patients with nasal symptoms attending a tertiary care center in Oman was found to be 7%. The diagnosis of AR is based on the patient history and is confirmed by skin testing. Many ‘knowledge gaps’ exist in relation to the pathogenesis, association of comorbidities, medical therapy (including immunotherapy), and surgical intervention in patients diagnosed with AR. Research in AR can be focused in the direction of these grey areas.
Disclosure
The authors declared no conflict of interest. No funding was received for this work.
references
- Al-Riyami BM, Al-Rawas OA, Al-Riyami AA, Jasim LG, Mohammed AJ. A relatively high prevalence and severity of asthma, allergic rhinitis and atopic eczema in schoolchildren in the Sultanate of Oman. Respirology 2003 Mar;8(1):69-76.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008 Apr; 63 Suppl 868-160.
- Al-Tamemi SH, Al-Shidhani AN, Al-Abri RK, Jothi B, Al-Rawas OA, Al-Riyami BM. The pattern of sensitisation to inhalant allergens in omani patients with asthma, allergic rhinitis and rhinoconjunctivitis. Sultan Qaboos Univ Med J 2008 Nov;8(3):319-324.
- Bhargava D, Bhargava K, Al-Abri A, Al-Bassam W, Al-Abri R. Non allergic rhinitis: prevalence, clinical profile and knowledge gaps in literature. Oman Med J 2011 Nov;26(6):416-420.
- Joe stephanie, Benson Aaron. Non-allergic rhinitis. In: Cummings Otolaryngology Head & Neck Surgery.(Eds,Cumming CW, Flint PW, Harker LA, et al.) Philadelphia, Pennsylvania: Mosby. 2005; p. 990-999.
- Blaiss MS. Quality of life in allergic rhinitis. Ann Allergy Asthma Immunol 1999 Nov;83(5):449-454.
- Druce HM. Allergic and non-allergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, eds. Allergy: Principles and Practice. 5th ed. St. Louis, Mo: Mosby Year-Book; 1998:1005-16.
- Siracusa A, Desrosiers M, Marabini A. Epidemiology of occupational rhinitis: prevalence, aetiology and determinants. Clin Exp Allergy 2000 Nov;30(11):1519-1534.
- Allergic Rhinitis and its Impact on Asthma (ARIA) 2010 revision. Geneva: World Health Organization (WHO); 2010 Dec 23. 153 p.
- Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al; Global Allergy and Asthma European Network; Grading of Recommendations Assessment, Development and Evaluation Working Group. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 2010 Sep;126(3):466-476.
- Okano M. Mechanisms and clinical implications of glucocorticosteroids in the treatment of allergic rhinitis. Clin Exp Immunol 2009 Nov;158(2):164-173.
- Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol 2011 Dec;128(6):1139-1150, e4.
- Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs 2007;67(6):887-901.
- Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy 2013 Feb;43(2):242-248.
- Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol 2012 Nov;130(5):1097, e2.
- Tsai JD, Chang SN, Mou CH, Sung FC, Lue KH. Association between atopic diseases and attention-deficit/hyperactivity disorder in childhood: a population-based case-control study. Ann Epidemiol 2013 Apr;23(4):185-188.
- Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol 2007;19:23-34.
- Arif Ali Kolethekkat, Roshna Rose Paul, Mary Kurien, Shyam Kumar, Rashid Al Abri, Kurien Thomas. Diagnosis of Adult Chronic Rhinosinusitis: Can Nasal endoscopy predict intrasinus disease? Oman Medical Journal 2013, (28); No 6: Nov. 427-431
- Mabry RL, Marple BF. Allergic rhinitis. In: Cummings Otolaryngology Head & Neck Surgery. (Eds, Cumming CW, Flint PW, Harker LA, et al.) Philadelphia, Pennsylvania: Mosby; 2005; p. 990-999.
- Scadding G, Durham S. Allergic rhinitis. In: Scott-Brown’s Otorhinolaryngology Head and neck Surgery. (Eds Gleeson M, Browning G, Burton MJ, et al.) Hodder Arnold Great Britain: Butterworth Co; 2008. p. 1387-1403.