|
Abstract
Objective: Neonates usually acquire Group B streptococcal infection vertically from the maternal birth canal during delivery. In January 2010, a Group B streptococcal outbreak investigation was conducted in response to an increased number of clinical specimens from our neonatal intensive care unit.
Methods: Microbiology laboratory records were reviewed to identify Group B streptococcal from specimens originating from the neonatal intensive care unit during December 2009 and January 2010. Patients from whom these specimens were collected were identified and their charts reviewed. Environmental samples to screen for Group B streptococcal were collected from the unit, clinical and environmental isolates were compared by pulsed field gel electrophoresis. Point prevalence screening was conducted twice before declaring the outbreak over.
Results: Pulsed field gel electrophoresis patterns of three clinical strains from six patients were indistinguishable. One environmental strain was isolated from one of the patients monitor, and had identical pulsed field gel electrophoresis pattern to that of the three clinical strains. Infection control measures were implemented in the neonatal intensive care unit and follow-up point prevalence screening identified no new cases.
Conclusions: Although poor infection control practice has been implicated in previous reports of nosocomial outbreaks of Group B streptococcal infection in neonatal intensive care units, our finding provides unique evidence that the environment can act as a reservoir of Group B streptococcal and play a key role in nosocomial transmission.
Keywords: Neonates; Group B streptococcus; Streptococcus agalactiae; Outbreak; NICU; Pulsed field gel electrophoresis (PFGE); Environment.
Introduction
Group B streptococcus (GBS), also known as Streptococcus agalactiae, causes severe disease in the neonatal period associated with significant morbidity and mortality.1 Early onset disease occurs during the first 6 days of life, while late onset can manifest any time from 7 days to 3 month of age. The frequency of early onset GBS infection has decreased substantially since the introduction of routine screening for maternal rectovaginal GBS colonization and the use of intrapartum antibiotic prophylaxis.2,3 However, the frequency of late onset infection, which is not affected by chemoprophylaxis, continues to cause significant morbidity and mortality in newborn.
Nosocomial outbreaks of late onset GBS infection are uncommon and have been attributed to direct and indirect infant-to-infant transmission via contaminated hands of nursery personnel.4-8 However, an epidemiological link between outbreak strains was not always found in reported clusters to support the theory of transmission.4,7,8 In this paper, we describe a genotypically-confirmed cluster of GBS infection in a level 3 neonatal intensive care unit (NICU) associated with environmental contamination by the same strain.
Methods
The NICU at the Hospital for Sick Children in Toronto, Canada is a 38-bed out-borne unit, providing tertiary medical and surgical services for neonates admitted from the greater Toronto area hospitals (figure 1). The typical census ranges from 30 to 34 patients.
On January 26th 2010, the microbiology laboratory informed Infection Prevention and Control of an increase in the number of clinical isolates of GBS from the NICU (5 unique patients isolates over 2 months). Three of the patients were still in the unit; one had been discharged and the other was deceased. Concurrently, a sixth child relocated to the referring hospital developed GBS bacteremia on return. Thus, including the latter case, there were six patients with GBS in this outbreak as compared to only one patient with GBS sepsis in 2009.
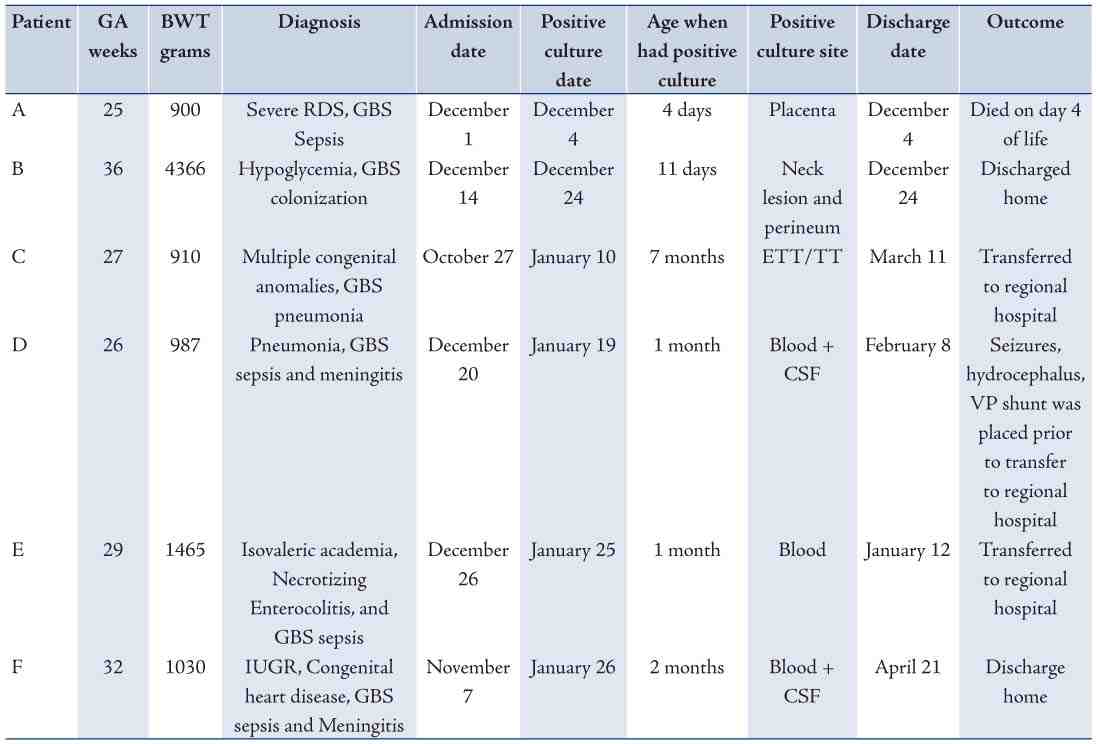
A case definition was established: any NICU patient from whom GBS was isolated from a clinical specimen since Dec 2009 and an outbreak investigation initiated. The clinical and laboratory records of these six patients were reviewed. Table 1 summarizes the patient characteristics and outcomes. The suspected index case (patient C) was transferred to our unit from a peripheral hospital, where the child’s respiratory secretions had previously grown GBS. The child required prolonged ventilation and frequent suctioning at our institution. Patient C shared a common room in the NICU with patients D and F, who both later developed GBS sepsis and meningitis, respectively (figure 1). Patient A died of early onset GBS sepsis; all the other patients had late onset disease. Patient E’s mother was GBS positive on antenatal screening; this child was in the unit for complex medical and surgical issues and then was transferred back to the community hospital on day 18 of life where he developed GBS sepsis on day 30 of life. In this outbreak, one child died (patient A), and another developed significant neurological sequelae (patient D) and required neurosurgical intervention for hydrocephalus.
Table 1: Demographic and outcome of neonates with positive GBS culture during outbreak.
NOTE: Patient C, D, and F shared same room in NICU and had indistinguishable GBS isolates by PFGE
Figure 1: NICU Design and Patients Location.
Patients with GBS, who were still in the NICU, were cohort with designated staff, and contact precautions were initiated. Environmental sampling was performed in the room, where three of the patients were residing. Ten sites in each patient specific zone (monitor, keyboard, mouse, IV pump, ventilator, lamp switch, parent chair- seat and back, thermometer and crib/front rail or incubator opening) were sampled, as well as ten non-patient specific sites (refrigerator handle, sink rim, diaper scale, phone, infant scale, two keyboards at nursing station, room handle in/out, and rocking chair). A total of 40 environmental samples were collected and sent to the microbiology laboratory for culture; only one site, the patient monitor above (patient D), grew GBS.
Extensive environmental cleaning of the room with the infected cohort was performed. A nursing role as room monitor was implemented to specifically manage cohort room precautions, monitor hand hygiene compliance, and minimize traffic flow. Education sessions reviewing basic infection prevention and control principles including hand hygiene were held for healthcare workers in the unit.
The NICU daily and monthly censuses from October 2009 to February 2010 were reviewed as a proxy for workload. Notably, in November 2009 the NICU reached maximum capacity (35 to 36 patients) for 7 days, had an average daily census of 33 patients, and 52 new admissions. This increased to 66 new patients in December. The average daily census fell to 29-30 patients during the months of January and February 2010. When the outbreak was declared over in February, there were no days where the unit had maximum census. Unfortunately, the nurse-patient ratios for the period were not available for review.
Four of the six clinical GBS isolates and the single environmental isolate were available for genotyping. Patient A’s and B’s isolates were not saved, as they were cultured from non-sterile sites before the outbreak was recognized. Pulsed-field gel electrophoresis of the GBS isolates was performed as per manufacturer’s protocol (BIO-RAD GenePath, Bio-Rad Laboratories (Canada) Ltd., Diagnostic Group, 2403 Guenette, Montreal, Quebec H4R 2E9). Briefly, the GBS strains were grown in PenAssay broth culture overnight and cells harvested were lysed within an agarose ‘plug’ with Lysozyme/Lysostaphin/Mutanolysin and then deproteinized. After a series of washes, endonuclease restriction was done using Sma I.9-11 The digested DNA was then separated in a 1% agarose gel by pulse-oriented electrophoresis. DNA fingerprints generated were interpreted by standard Tenover's criteria.12
Results
By PFGE analysis, three clinical isolates were indistinguishable (figure 2A). All three patients, from whom those isolates were cultured, were admitted to the same room in the NICU. Patient E’s GBS isolate PFGE differed from the outbreak pattern (Figure 2B); this patient had not shared a room with the other infected patients. Only one of the 40 environmental surfaces sampled, from patient D’s monitor, grew GBS. This environmental strain was indistinguishable from the strains of the patients, who had been housed in the same room (Figure 2A).
Two unit wide point prevalence screens (stool and respiratory secretions if intubated) were performed in February. No new patients with GBS were identified (February 4th and 10th) and the outbreak was declared over.
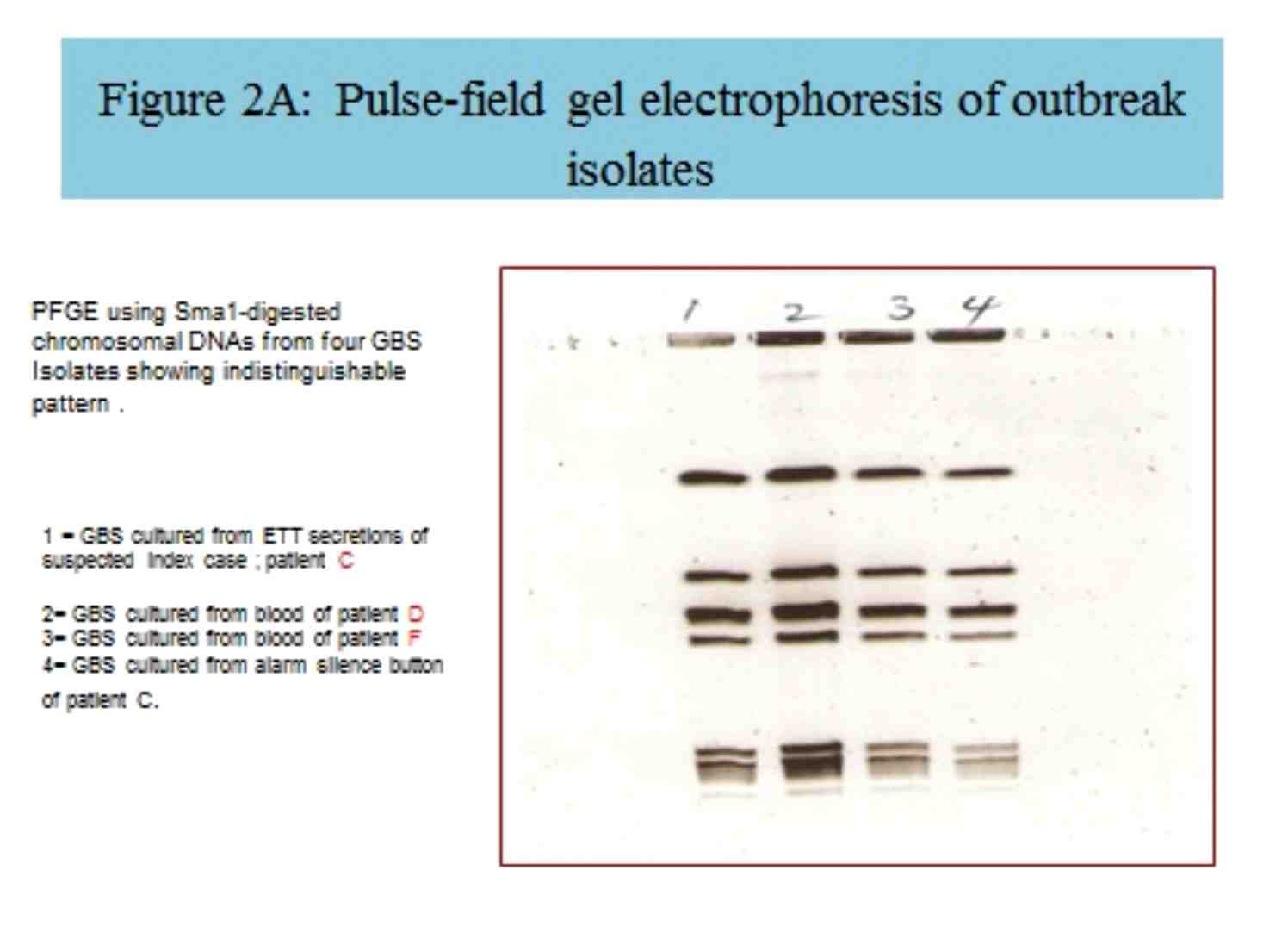
Figure 2A: Pulse-field gel electrophoresis of outbreak isolates.
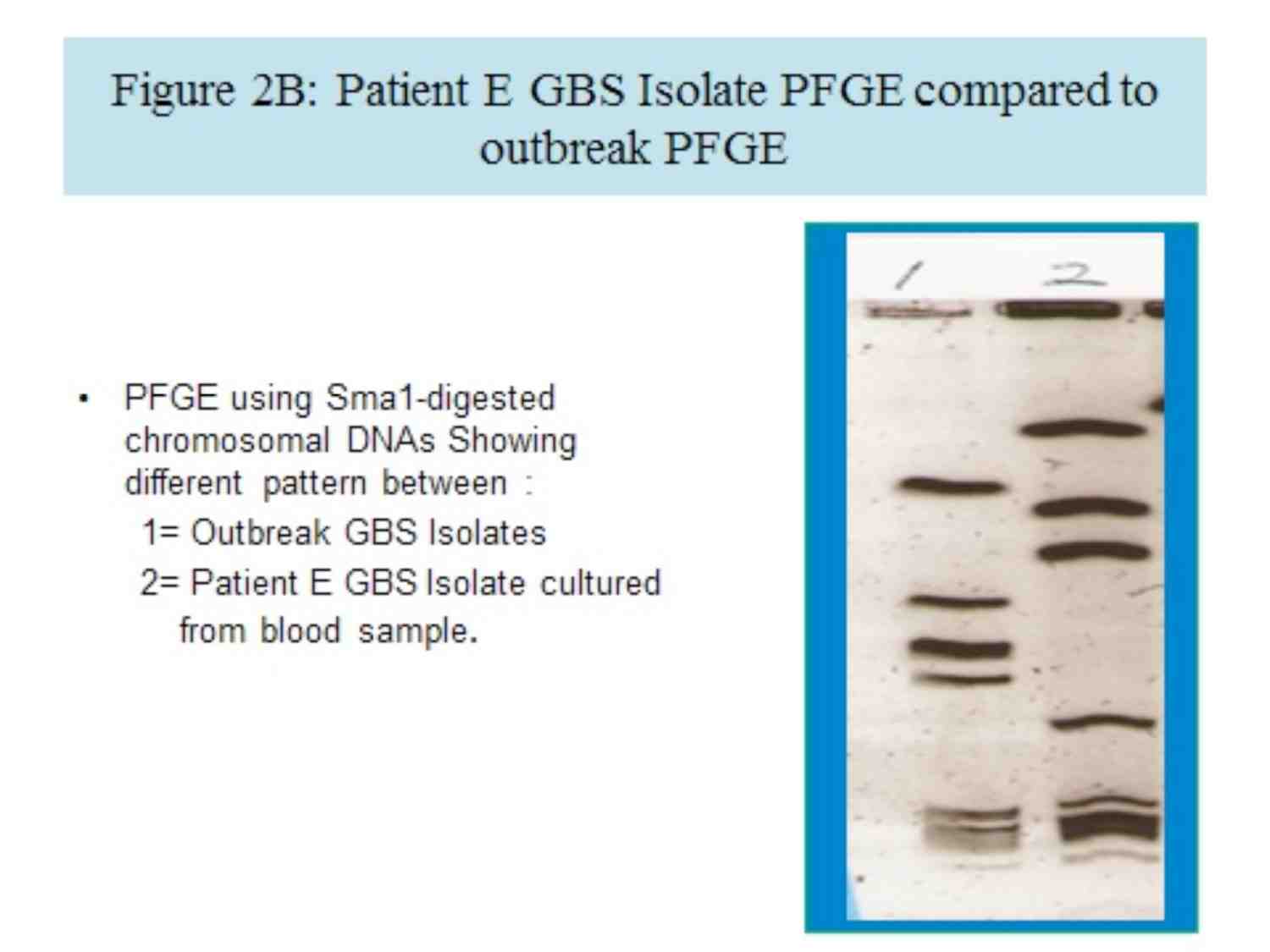
Figure 2B: Patient E GBS Isolate PFGE compared to outbreak PFGE.
Discussion
While previous reports have hypothesized poor infection control practices as the cause of late onset GBS outbreaks in neonates, this is the first to demonstrate an environmental reservoir of GBS during an outbreak. We suspect that during the course of care, a healthcare worker handled the monitor without performing proper hand hygiene and the medical device was not cleaned prior to caring for a different patient.
Environmental contamination is increasingly being appreciated as a reservoir for pathogens involved in outbreaks. Patient monitors and other high touch surfaces have been implicated as the source. Although the importance of environmental cleaning for preventing and controlling outbreaks of infection in the healthcare setting is appreciated, cleaning staff may be uncomfortable cleaning electronic equipment.13,14 Failure to clean properly, rather than the actual procedure or product used for cleaning, has been shown, for example, to be responsible for persistence of surface contamination with vancomycin resistant enterococci.15
This outbreak spanned the Christmas and New Year period, which is usually characterized by relative understaffing and an increase workload per health care worker. In a previous Study by Pittet et al; high workload was reported to be associated with a high risk for cross-transmission to the patients.16 It was showed that noncompliance with hand washing was higher in intensive care than in internal medicine units, during procedures that carry a high risk for contamination (before intravenous care, before respiratory care, and care between a dirty and a clean body site), and when the intensity of patient care was high.17 The consequences of poor infection control practices are magnified in a high risk patient population (eg premature neonates who lack maternal antibody) exposed to a virulent pathogen such as GBS. Despite lack of robust measures of workload, we still posit that seasonal staffing pressures in a full NICU were important risk factors in this outbreak.
Neonatal sepsis remains the leading cause of mortality especially in the preterm babies who needs to be admitted to NICU for long time. The profile of organisms differ between different units but GBS continue to be in the top of the list.18,19 GBS infection in neonates carries significant morbidity and mortality. Intrapartum antibiotic prophylaxis has helped make early onset disease preventable, and our study supports the hypothesis that good infection control practices, i.e. hand hygiene and environmental cleaning can render healthcare associated late onset GBS disease preventable as well. These cornerstones of infection prevention and control remain dominant in the ongoing efforts to eliminate all hospital-associated infection.
Conclusion
Although nosocomial transmission of GBS has been implicated in previous outbreaks in newborn units, our finding supports indirect transfer of GBS from patient to patient via contaminated hands of health care workers with involvement of a contaminated environmental intermediary. Hand hygiene and environmental cleaning remain the cornerstones of infection prevention and control; therefore, it must be practiced consistently particularly in busy times.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
1. Guilbert J, Levy C, Cohen R, Delacourt C, Renolleau S, Flamant C; Bacterial meningitis group. Late and ultra late onset Streptococcus B meningitis: clinical and bacteriological data over 6 years in France. Acta Paediatr 2010 Jan;99(1):47-51.
2. Centers for Disease Control and Prevention (CDC). Perinatal group B streptococcal disease after universal screening recommendations–United States, 2003-2005. MMWR Morb Mortal Wkly Rep 2007 Jul;56(28):701-705.
3. Centers for Disease Control and Prevention (CDC). Prevention of Perinatal Group B Streptococcal Disease Revised Guidlines. MMWR Morb Mortal Wkly Rep 2010;59(10):1-32.
4. Steere AC, Aber RC, Warford LR, Murphy KE, Feeley JC, Hayes PS, et al. Possible nosocomial transmission of group B streptococci in a newborn nursery. J Pediatr 1975 Nov;87(5):784-787.
5. Aber RC, Allen N, Howell JT, Wilkenson HW, Facklam RR. Nosocomial transmission of group B streptococci. Pediatrics 1976 Sep;58(3):346-353.
6. Easmon CS, Hastings MJ, Clare AJ, Bloxham B, Marwood R, Rivers RP, et al. Nosocomial transmission of group B streptococci. Br Med J (Clin Res Ed) 1981 Aug;283(6289):459-461.
7. MacFarquhar JK, Jones TF, Woron AM, Kainer MA, Whitney CG, Beall B, et al. Outbreak of late-onset group B Streptococcus in a neonatal intensive care unit. Am J Infect Control 2010 May;38(4):283-288.
8. Weems JJ Jr, Jarvis WR, Colman G. A cluster of late onset group B streptococcal infections in low birth weight premature infants: no evidence for horizontal transmission. Pediatr Infect Dis 1986 Nov-Dec;5(6):715-717.
9. Miranda AG, Singh KV, Murray BE. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol 1991 Dec;29(12):2752-2757.
10. Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol 1990 Sep;28(9):2059-2063.
11. Gordillo ME, Singh KV, Baker CJ, Murray BE. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol 1993 Jun;31(6):1430-1434.
12. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995 Sep;33(9):2233-2239.
13. Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 2007 Jun;65(Suppl 2):50-54.
14. Carling PC, Parry MF, Bruno-Murtha LA, Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit Care Med 2010 Apr;38(4):1054-1059.
15. Hota B, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Interventional evaluation of environmental contamination by vancomycin-resistant enterococci: failure of personnel, product, or procedure? J Hosp Infect 2009 Feb;71(2):123-131.
16. Pittet D, Simon A, Hugonnet S, Pessoa-Silva CL, Sauvan V, Perneger TV. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004 Jul;141(1):1-8.
17. Pittet D, Mourouga P, Perneger TV. Compliance with handwashing in a teaching hospital. Infection Control Program. Ann Intern Med 1999 Jan;130(2):126-130.
18. Gupta B, El Amin E. Changing patterns of blood borne sepsis in special care baby unit, khoula hospital. Oman Med J 2010 Apr;25(2):100-103.
19. Abdellatif M, Ahmed M, Bataclan MF, Khan AA, Al Battashi A, Al Maniri A. The patterns and causes of neonatal mortality at a tertiary hospital in oman. Oman Med J 2013 Nov;28(6):422-426.
|