|
Abstract
Objective: Genetic variants of the melanocortin-4 receptor gene (MC4R), agouti related protein (AGRP) and proopiomelanocortin (POMC) are reported to be associated with obesity. Therefore, the aim of this study is to examine MC4R rs17782313, MC4R rs17700633, AGRP rs3412352 and POMCrs1042571 for any association with obesity in North Indian subjects.
Methods: The variants were investigated for association in 300 individuals with BMI ≥30 kg/m2 and 300 healthy non-obese individuals BMI <30 kg/m2. The genotyping were analyzed by Taqman probes. The statistical analysis was performed by the SPSS software, ver.19 and p≤0.05 was considered statistically significant.
Results: The genotypes of MC4R rs17782313 and POMC rs1042571 were significantly associated with obesity (C), (p=0.02; OR=1.7 and p=0.01; OR=1.6, respectively); however, MC4Rrs17700633 (p=0.001; OR=0.55) was associated with low risk. In addition, AGRPrs3412352 (p=0.93; OR=0.96) showed no association with obesity (BMI ≥30 kg/m2) in North Indian subjects.
Conclusion: This study provides the report about the significant association of MC4R (rs17782313) and POMC (rs1042571) with morbid obesity (BMI ≥30 kg/m2), but MC4R (rs17700633) and AGRP (rs34123523) did not show any association with obesity in the studied North Indian population.
Keywords: Obesity; Genetic variants; Genotype; Phenotype; MC4R; AGRP; POMC.
Introduction
Obesity and the associated risk of cardiovascular disease is a rising global health burden. Numerous variations in genes may contribute to the pathogenesis of obesity. The physiological pathways related to appetite are complex and involve the mixing of short-term satiety signals from the gut to the brain along with longer-term homeostatic systems which grip the integrated signaling of POMC, AGRP, and MC4R systems and risk an individual to obesity.1
Monogenic forms of obesity have been recognized with mutations in the gene encoding the melanocortin-4 receptor (MC4R) being the most widespread.2 MC4R is expressed in the central nervous system and the encoded protein is involved in appetite regulation. MC4R gene rs17782313 polymorphism is significantly associated with obesity risk among both European adults and children.3
Agouti related protein (AGRP) is an evolutionary conserved gene mainly located in ARC neurons that co-express neuropeptide Y (NPY) and is also a strong negotiator of regulation of energy balance.4 AGRP acts as an endogenous inverse antagonist of melanocortin 4 receptor (MC4R) gene. A few single nucleotide polymorphisms (SNPs) have previously been known in the AGRP gene being associated with energy homeostasis disorder phenotypes. SNPs in the promoter region of AGRP, -3019G>A and -38C>T have been associated with obesity in individuals of "African" origin apart from "Caucasians".5
Melanocortin signaling in the hypothalamus plays a vital role in the control of energy homeostasis. Intracellular post translation of the POMC pro-peptide by pro-hormone convertase 2 leads to the production of α, β, and γ melanocyte-stimulating hormones. These peptides signal to downstream target neurons in the lateral hypothalamus that express the melanocortin receptor MC4R with consequential decrease in food intake and increase in energy expenditure.6 Rare mutations in the POMC gene (Cytogenetic Location: 2p23.3) cause early-onset obesity in humans though the influence of common polymorphisms in POMC on obesity phenotypes in less extreme individuals is indistinct.7
Till now, there are many well-documented obesity SNPs but this work focused on these four physiological pathway related essential SNPs; MC4R rs17700633, MC4R rs17782313, AGRP rs3412352 and POMC rs1042571 with obesity BMI ≥30 kg/m2 because there are no reports available of any association study conducted among North Indian individuals. Therefore, the aim of this study was to examine their influence in advancement of obesity (BMI ≥30 kg/m2) in the general population of Northern India.
Methods
All individuals in the study were of north Indian origin and the population was homogeneous with regard to ethnic background. Individuals other than North Indian origin were excluded. Prior informed written consent was obtained from each participant and the identity of all participants was kept confidential. The study was carried out according to the local Ethics Committee. The study conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (18 July 1964). All study participants were subjected to a thorough screening program that included assessment of a detailed personal and family history, physical examination and determination of anthropometric measurements. Case-control studies of obesity were performed on 600 individuals selected befitting the strict inclusion criteria.
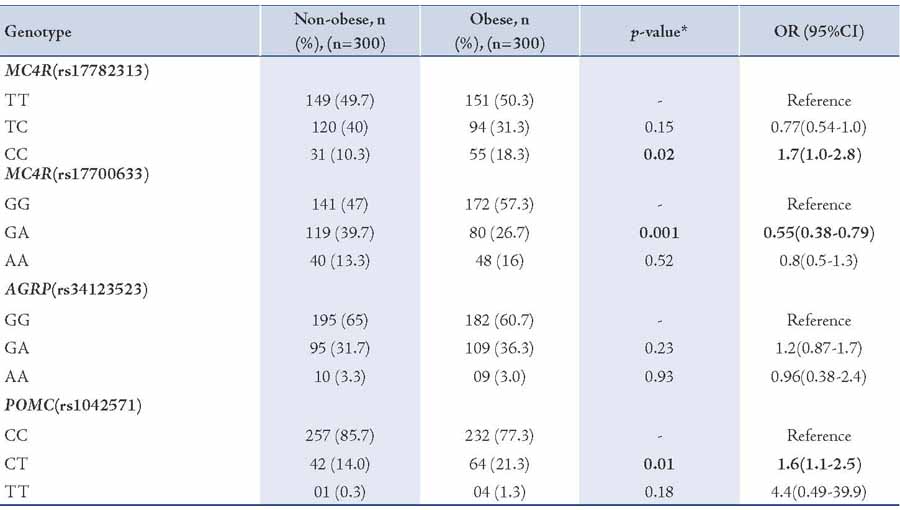
Body weight and height were measured with individuals wearing light clothing. Waist circumference was measured midway between the iliac crest and the lower costal margin along with hip circumference (Table 1). Blood samples were taken and serum was isolated for lipid profile estimation via kit (ERBA diagnostics Mannheim GmbH, Germany).
Genotyping of MC4R rs17782313, MC4R rs17700633, AGRP rs3412352 and POMC rs1042571 was performed using Taqman® allelic discrimination (Applied Biosystems®). Genotype distributions obeyed Hardy-Weinberg equilibrium (p>0.05) in the non-obese individuals. Genotype and allele distribution was compared between obese and non-obese subjects using X2 test. The independent segregation of alleles was tested for the Hardy-Weinberg equilibrium (HWE), comparing the observed genotype frequencies with those expected (X2 test). For case-control studies, differences in genotype distributions were calculated applying an additive logistic regression model adjusted for sex and age. All analyses of association between genotype and phenotypes were conducted using the Statistical Package for Social Sciences software (SPSS) ver.19 and p≤0.05 was considered statistically significant.
Results
Genotypic data for 300 obese and 300 non-obese subjects were analyzed. Four variants: MC4R (rs17782313, rs17700633), AGRP (rs34123523) and POMC (rs1042571) were genotyped in the North Indian population. Anthropometric and clinical characteristics of the subjects are provided in Table 1.
The results are depicted in Table 2. The analysis revealed that the CC genotype of MC4R rs17782313, (p=0.02; OR=1.7) and CT genotype of POMC rs1042571, (p=0.01; OR=1.6) SNPs were significantly associated with obese individuals (BMI ≥30 kg/m2) when compared with non-obese individuals (BMI <30 kg/m2). The GA heterozygote of MC4R rs17700633, (p=0.001; OR=0.55) showed association but at lower risk. However, no association of AGRP rs34123523 (p=0.23; OR=1.2) was seen with BMI ≥30 kg/m2.
Table 1: Anthropometrical and lipid profile measurements of non-obese (BMI <30 kg/m2) and obese (BMI ≥30 kg/m2) subjects.
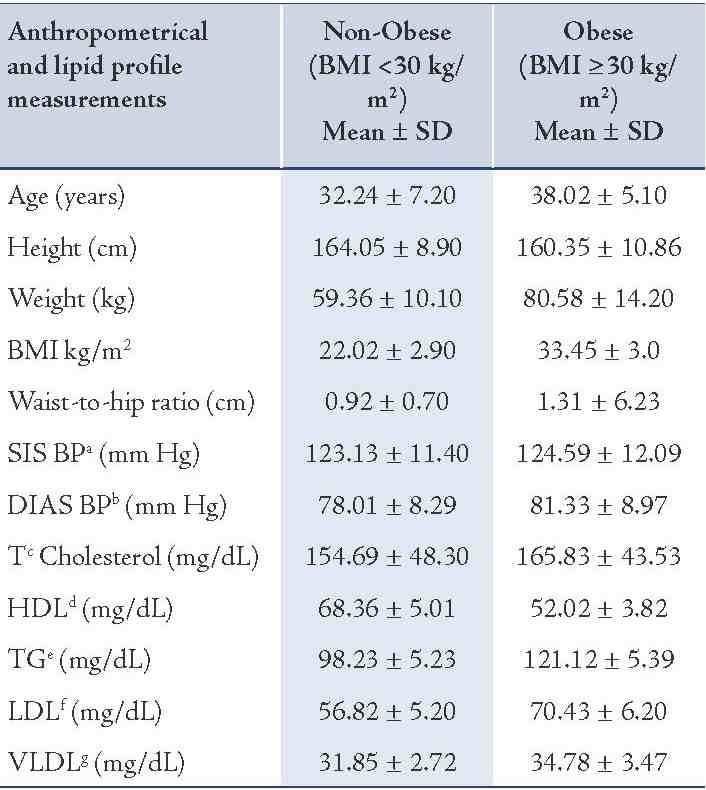
Table 2: Frequency distribution of genotypes in MC4R rs17782313, MC4R rs17700633, AGRP rs3412352 and POMC rs1042571 for association with obesity BMI ≥30 kg/m2 in North Indian individuals.
Discussion
The current study reports the association of the SNPs MC4R (rs17782313, rs17700633), AGRP (rs34123523), and POMC (rs1042571) with BMI ≥30 kg/m2. To the authors’ knowledge, this is the first report of associations of these SNPs with obesity in a North Indian population. It was found that the common obesity-associated MC4R rs17782313 variant genotype was significantly associated with obesity (BMI ≥30 kg/m2; p=0.02; OR=1.7) among the north Indian population. The association of the SNP MC4R rs17782313 with obesity has previously been investigated in "European-American" children and studies have reported significant associations.8 The SNP was also found significantly associated with obesity in adult Chinese,9 central obesity in Chinese children,10 Danish,11 "African" and "European-American" populations.12
GWAS studies have also identified the common polymorphism rs17782313 associated with obesity risk in Europeans (OR = 1.12; 95% CI = 1.08-1.16; p=5.2×10-9).3 Among all the GWAS-identified obesity-associated loci, MC4R and POMC variants have influence on obesity and contribute to the variance in body mass index (BMI) in Europeans and East Asians.13 It is also well established that the MC4R variant genotype of rs17782313 were associated with higher intake of energy and also higher percentage of energy from fatty diets.11 Investigations of the association between genetic variation and obesity traits in Indian populations have found MC4R variant rs17782313 to be associated but weakly with weight and hip circumference (p<0.05).14 Rare mutations in the MC4R gene were earlier associated with binge eating, excessive hunger, hyperphagia and food-seeking actions.15-17 Therefore, findings are in agreement that MC4R rs17782313 plays a role in predisposition to obesity BMI ≥30 kg/m2 in view of the fact that it is expressed in the brain and is part of the melanocortin pathway controlling food intake and energy expenditure.17
In the present study, association with MC4R rs17700633 and obesity BMI ≥30 kg/m2 (p=0.001; OR=0.55) was observed but at low risk. The cause for the discrepancy among the current study and the previous studies was not clear. Other studies have found significant association between MC4R rs17700633 and BMI ≥30 kg/m2 at high risk; but on the other hand, studies have also reported that there is no association of MC4R rs17700633 with dietary intakes, adiposity measures or obesity traits.11 Loss or gain of AGRP gene may result in inadequate adaptive behavioral responses to environmental events, such as stress, and may potentially contribute to the development of eating disorders.18 Studies in two ethnic specific polymorphisms suggest that slight differences in food preference in carriers of the rare AGRP alleles (T/T and Ala67Thr in blacks and whites, respectively) could over time result in lower adiposity.19 However, no association of AGRP rs11575892 (p=0.23; OR=0.96) with obesity phenotype was observed.
In this study of POMC rs1042571 CT heterozygote and BMI ≥30 kg/m2 was significantly associated (p=0.01; OR=1.6) with higher BMI ≥30 kg/m2 and results are in agreement with those reported by Krude et al. in 2003,7 who recognized homozygosity or compound heterozygosity for mutations in the POMC gene. The investigators noted that all of the heterozygous persons in their study had BMIs in the high, normal to overweight ranges. This fact supports the notion that defects in POMC are the cause of pro-opiomelanocortinin deficiency and affected individuals may present early-onset to obesity.
In the present study, the rationale for such associations of individual SNPs that make very little variance and some discrepancy when compared to previous studies on MC4R rs17700633 and AGRP rs11575892 in other populations is possibly, to a certain degree, secondary to the difference in the population characteristics based on social-environmental influences,20 socioeconomic status (SES) i.e., occupational status, education, income,21 and non-exercise activity thermogenisis (NEAT),22 resulting to decreased physical activity and thus decreased energy expenditure being negatively associated with BMI,23-25 along with food preference and obese eating style,26-29 which differentiate lean and obese individuals' eating behaviors and their risk to obesity. Nevertheless this is the first study in relation to MC4R (rs17782313, rs17700633), AGRP (rs34123523) and POMC (rs1042571) and their association with obesity (BMI ≥30 kg/m2) in North Indian population.
In view of the fact that obesity is caused by perturbations of the balance among food intake and energy expenditure all along with social-environmental factors which are regulated by a complex physiological system that requires the incorporation of several peripheral signals and central coordination in the brain, the outcome of the study implies that polymorphisms in MC4R, and POMC have a function in the regulation of food intake, energy expenditure and preference for specific food items which may result in obesity.
Slight changes in expression of these genes because of mutations in regulatory regions collectively with feeding behavior, food preference, lifestyles and environmental exposures may therefore play a role in the multigenic and pleotropic susceptibility for obesity, providing valuable information on gene regulation making them suitable candidates for testing in large-scale case-control studies of genetic susceptibility towards development of obesity and could provide a diagnostic marker for obesity (BMI ≥30 kg/m2) in Indian populations as these genes possibly play the key role in contemporary lifestyles in the current obesity outbreak being involved in physiological pathways related to appetite.
Conclusion
In summary, study on the association of four physiologically important SNPs MC4R rs17782313, MC4R rs17700633, AGRP rs3412352, and POMC rs1042571 with obesity (BMI ≥30 kg/m2) in North Indian population is presented in this report for the first time suggesting that naturally occurring mutations in MC4R, AGRP and POMC can make an individual be at risk of obesity.
Acknowledgments
The authors acknowledge all the participating subjects for their support and cooperation in carrying out the study as well as the Department of Biotechnology (DBT), Government of India, New Delhi, for the financial support to carry out this research work.
References
1. Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science 2005 Mar;307(5717):1909-1914.
2. Larsen LH, Echwald SM, Sørensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 2005 Jan;90(1):219-224.
3. Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, et al; Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial; KORA; Nurses’ Health Study; Diabetes Genetics Initiative; SardiNIA Study; Wellcome Trust Case Control Consortium; FUSION. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 2008 Jun;40(6):768-775.
4. Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, et al. The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol 2004 Mar;21(3):563-579.
5. Mayfield DK, Brown AM, Page GP, Garvey WT, Shriver MD, Argyropoulos G. A role for the Agouti-Related Protein promoter in obesity and type 2 diabetes. Biochem Biophys Res Commun 2001 Sep;287(2):568-573.
6. Yeo GS, Farooqi IS, Challis BG, Jackson RS, O’Rahilly S. The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. QJM 2000 Jan;93(1):7-14.
7. Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, et al. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab 2003 Oct;88(10):4633-4640.
8. Zobel DP, Andreasen CH, Grarup N, Eiberg H, Sørensen TI, Sandbaek A, et al. Variants near MC4R are associated with obesity and influence obesity-related quantitative traits in a population of middle-aged people: studies of 14,940 Danes. Diabetes 2009 Mar;58(3):757-764.
9. Cheung CY, Tso AW, Cheung BM, Xu A, Ong KL, Fong CH, et al. Obesity susceptibility genetic variants identified from recent genome-wide association studies: implications in a chinese population. J Clin Endocrinol Metab 2010 Mar;95(3):1395-1403.
10. Xi B, Cheng H, Shen Y, Chandak GR, Zhao X, Hou D, et al. Study of 11 BMI-associated loci identified in GWAS for associations with central obesity in the Chinese children. PLoS One 2013;8(2):e56472.
11. Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet 2008 Nov;17(22):3502-3508.
12. Liu G, Zhu H, Dong Y, Podolsky RH, Treiber FA, Snieder H. Influence of common variants in FTO and near INSIG2 and MC4R on growth curves for adiposity in African- and European-American youth. Eur J Epidemiol 2011 Jun;26(6):463-473.
13. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al; MAGIC; Procardis Consortium. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010 Nov;42(11):937-948.
14. Taylor AE, Sandeep MN, Janipalli CS, Giambartolomei C, Evans DM, Kranthi Kumar MV, et al. Associations of FTO and MC4R Variants with Obesity Traits in Indians and the Role of Rural/Urban Environment as a Possible Effect Modifier. J Obes 2011;2011:307542.
15. Mergen M, Mergen H, Ozata M, Oner R, Oner C. A novel melanocortin 4 receptor (MC4R) gene mutation associated with morbid obesity. J Clin Endocrinol Metab 2001 Jul;86(7):3448-3451.
16. Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 2003 Mar;348(12):1096-1103.
17. Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol 2006 Dec;149(7):815-827.
18. Vink T, Hinney A, van Elburg AA, van Goozen SH, Sandkuijl LA, Sinke RJ, et al. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry 2001 May;6(3):325-328.
19. Loos RJ, Rankinen T, Rice T, Rao DC, Leon AS, Skinner JS, et al. Two ethnic-specific polymorphisms in the human Agouti-related protein gene are associated with macronutrient intake. Am J Clin Nutr 2005 Nov;82(5):1097-1101.
20. Barnett E, Casper M. A definition of "social environment". Am J Public Health 2001 Mar;91(3):465.
21. Ball K, Crawford D. Socioeconomic status and weight change in adults: a review. Soc Sci Med 2005 May;60(9):1987-2010.
22. Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science 2005 Jan;307(5709):584-586.
23. Parsons TJ, Power C, Manor O. Physical activity, television viewing and body mass index: a cross-sectional analysis from childhood to adulthood in the 1958 British cohort. Int J Obes (Lond) 2005 Oct;29(10):1212-1221.
24. Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr 2005 Jul;82(1)(Suppl):226S-229S.
25. Wareham NJ, van Sluijs EM, Ekelund U. Physical activity and obesity prevention: a review of the current evidence. Proc Nutr Soc 2005 May;64(2):229-247.
26. Ernersson A, Lindström T, Nyström FH, Frisman GH. Young healthy individuals develop lack of energy when adopting an obesity provoking behaviour for 4 weeks: a phenomenological analysis. Scand J Caring Sci 2010 Sep;24(3):565-571.
27. Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep 2010 Jun;33(6):753-757.
28. Wronka I, Suliga E, Pawliñska-Chmara R. Evaluation of lifestyle of underweight, normal weight and overweight young women. Coll Antropol 2013 Jun;37(2):359-365.
29. Veghari G, Sedaghat M, Banihashem S, Moharloei P, Angizeh A, Tazik E, et al. Trends in waist circumference and central obesity in adults, northern iran. Oman Med J 2012 Jan;27(1):50-53.
|