| |
Abstract
Objective: To evaluate the usefulness of Tru-Cut biopsy (TCB) in the diagnosis of breast lesions.
Methods: An observational non-interventional cross-sectional review was performed of all TCB reports of a mass or lesion observed in patients admitted between January 2008 and December 2010, at King Khalid University Hospital, Riyadh, Saudi Arabia.
Results: A total of 275 trucut biopsies were performed during the study period. Histopathology showed 92 (33.5%) malignant lesions, 177 (64.3%) benign lesions and 6 (2.2%) suboptimal or suspicious lesions. Repeat trucut biopsies were done in 29 (16.4%) of the benign cases, 12 (13%) of the malignant cases, and for 6 inconclusive specimens which showed 4 of the 29 benign cases to be malignant lesions, and 5 of the 6 inconclusive cases were also malignant lesions. All 12 malignant cases that had repeat trucut biopsy had a confirmed diagnosis of malignancy. Trucut biospy had a sensitivity of 95.1%, specificity of 100%, positive predictive value of 100%, negative predictive value of 97.2%, and an overall diagnostic accuracy of 98.2%.
Conclusion: Trucut biopsy is an accurate alternative to fine needle aspiration cytology in the diagnosis of breast lesions with a high diagnostic accuracy of 98.2%.
Keywords: Breast Cancer; Tru-Cut Biopsy; Saudi Arabia.
Introduction
Breast cancer is the most common type of cancer in Saudi Arabian women.1 The Saudi National Cancer Registry (NCR) reported an increasing incidence of breast cancer among women of all ages from 10.2% in 2000 to 24.3% in 2005.1 To ensure an accurate diagnosis, the combination of a good clinical eye, high-quality imaging, and appropriate pathological techniques is important. For several years, fine needle aspiration cytology (FNAC) was the most practiced method for the pathological diagnosis of breast masses. FNAC became popular because of its accuracy, cost effectiveness, and ease of use.2-4
The advent of core needle or Tru-Cut biopsy (TCB) in the new millennium has resulted in many surgeons switching to TCB since it provides a sufficient amount of tissue for pathologists to make an accurate histological diagnosis. It is now the initial investigation method of choice for pre-operative diagnosis of breast lesions. Some studies have suggested that TCB is superior to FNAC to some extent. The sensitivity of FNAC increases when TCB biopsy is also performed.5 TCB can provide all the necessary details to guide both the surgeon and the oncologist in designing an appropriate therapeutic strategy for the management of patients with breast masses. This report is aimed at evaluating the diagnostic usefulness of TCB in the diagnosis of breast masses in patients admitted to King Khalid University Hospital, Riyadh, Saudi Arabia, from January 2008 to December 2011.
Methods
A cross-sectional study of all histopathological reports of patients who had undergone TCB of a breast mass or lesion between January 2008 and December 2010 at King Khalid University Hospital, Riyadh, Saudi Arabia, was undertaken. Histomorphological features were classified as benign or malignant based on the 2003 World Health Organization classification of tumors of the breast.6
TCB was performed using a Tru-Cut gun with an 18-gauge needle. After manual localization and immobilization of the lesion under complete aseptic technique, a 2% lignocaine-infiltrating anesthetic was administered and a skin incision was performed. A biopsy specimen was obtained by means of 4 successive insertions with different angulations of the needle into the lesion’s core. After immediate immersion of the specimen in a fixative, its quantity and quality were judged and it was sent to the histopathology department. All breast masses were clinically palpable. The histopathological reports of the TCB specimens were compared with the histopathological reports of follow-up procedures including surgical procedures like mastectomy, excision biopsy, or wide local excision.
Each TCB diagnosis was matched with the histopathology results and labeled as follows: true positive (TP) when positive TCB result for malignancy is confirmed in the histological study of the post-surgical specimen; false positive (FP) when positive TCB result for malignancy is not confirmed in the histological study of the post-surgical specimen; true negative (TN) when negative TCB result for malignancy is obtained and no carcinoma in the histological study of the post-surgical specimen is found; and false negative (FN) when negative TCB result for malignancy is obtained but a carcinoma is detected in the histological study of the post-surgical specimen.7
Sensitivity (SN) was measured as the proportion of patients with an associated carcinoma and a positive TCB result for malignancy. The formula used for sensitivity was; SN = TP/ (TP + FN). While Specificity (SP) was based on the proportion of patients without associated carcinoma and a negative TCB result for malignancy. The formula used for specificity was SP= TN/ (TN + FP). Positive predictive value (PPV) was considered as the proportion of patients with a positive TCB result and histological confirmation of malignancy of the post-surgical specimen. The formula used for positive predictive value was PPV = TP/ (TP + FP). Negative predictive value (NPV) was defined as the proportion of patients with negative TCB results and without carcinoma in the histological study of the post-surgical specimen. The formula used for negative predictive value was NPV = TN/ (TN + FN). Diagnostic accuracy (DA) was based on the proportion of patients diagnosed correctly using the diagnostic test. The formula used for diagnostic accuracy was DA = (TP + TN)/ (FP + FN + TP + TN).
All data were verified prior to being entered into a Microsoft Excel 2007 worksheet. Statistical analyses were done using Predictive Analysis Software version 18.0 (SPSS; IBM, Chicago, IL, USA). Data are presented as mean, standard deviation and percentage distribution. Calculations of specificity, sensitivity, PPV, and NPV were done using the formulas provided above.
Results
Two-hundred and seventy-five TCB procedures were performed between January 2008 and December 2010 at King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia. The median age of patients was 43 years old (range: 15 to 91 years). The histopathological diagnosis of the TCB specimen showed 177 cases (64.4%) to be benign lesions, 92 (33.5%) as malignant lesions and 6 (2.2%) as inconclusive, (Table 1). A repeat TCB was done on 29 (16.4%) of the benign cases, 12 (13.0%) of the malignant cases and also on the 6 inconclusive TCB specimens. Repeat TCB was done 1.0 ± 0.2 years after the initial TCB.
Of the 29 benign cases in which repeat TCB was performed; 25 (86.2%) turned out to be benign, whereas 4 cases (13.8%) were found to be malignant cases. The results also showed that of the 12 malignant cases in which repeat TCB was performed, all 12 (100%) were found to be malignant on the second TCB. The 6 inconclusive specimens were found to be malignant in 5 cases (83.3%) and 1 (16.7%) was revealed to be a benign lesion on repeat TCB. The indication for those repeat TCBs was inadequate samples or for confirmation of a diagnosis rendered on a scanty or hypocellular specimen. Final diagnosis of TCB specimen after repeat TCB showed a total of 174 benign cases (63.3%) and 101 (36.7%) malignant cases. (Table 1)
Based on the final histopathological diagnosis of the TCB specimens, there were 97 (35.3%) true-positive cases, 173 (62.9%) true-negative cases, 5 (1.8%) false-negative cases and no false-positive cases. TCB exhibited a sensitivity of 95.1%, 100% specificity, PPV of 100%, NPV of 97.2%, and an overall DA of 98.2%. It also provided a definitive histological type and grade that correlated with the final histopathology report in 74 of the 96 (77.1%) malignant cases.
Table 1: Histopathological findings of TCB vs. post-surgical samples of breast lesions.
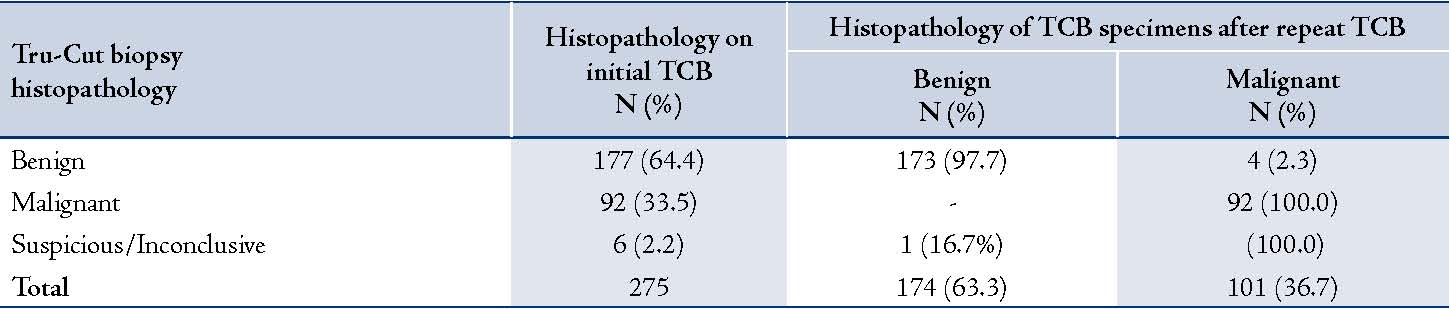
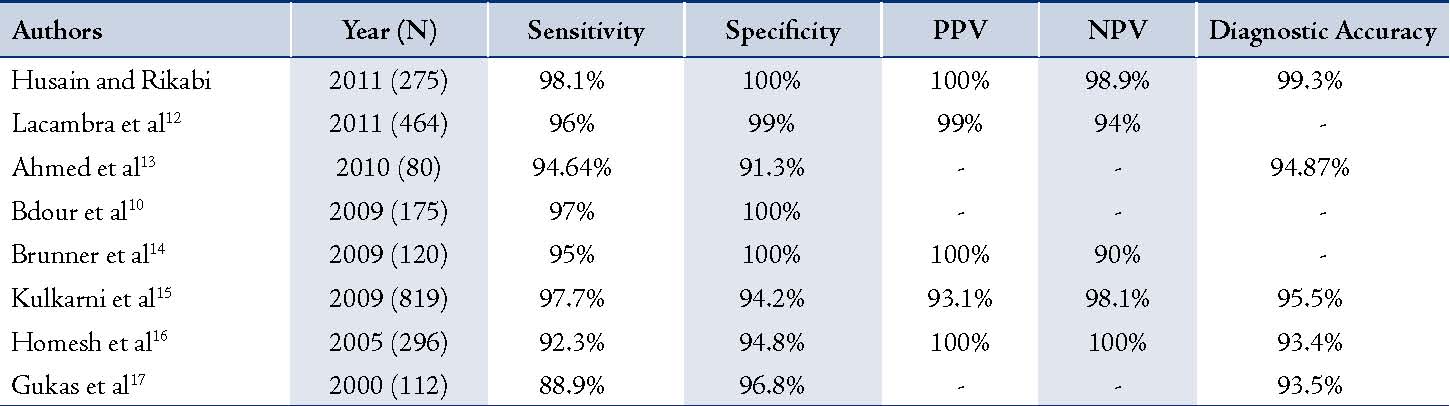
Table 2: Comparison of studies conducted to determine the usefulness of TCB in the diagnosis of breast masses.
PPV: positive predictive value; NPV: negative predictive value
Discussion
Several studies have determined the usefulness of FNAC in the diagnosis of breast masses with high sensitivity of 84-98% and specificity of >99%.5,8,9 On the other hand, some recent studies have proven that TCB is superior to FNAC.10,11 The results of this retrospective study suggest that TCB is an accurate, reliable and a safe method of establishing the diagnosis of cancer in patients with breast lesions. Our results yielded a high sensitivity of 95.1% with 100% specificity, and a PPV, NPV, and DA of 100%, 97.2%, and 98.2%, respectively. In fact, there were no false positive results. This means that TCB provides a breast cancer diagnosis with a high degree of confidence. Any patient with TCB results that are consistent with breast carcinoma should be referred to surgery and oncology for immediate management and treatment. Compared to several studies that were conducted on the diagnostic usefulness of TCB in the diagnosis of breast masses,10,12-17 the current findings have surpassed those previously reported with regards to diagnostic accuracy (DA). (Table 2)
Our study showed four cases of false negatives which lowered the diagnostic accuracy of the result to 98.1%. All five cases showed a benign lesion on first TCB, but turned out to be malignant lesions when a repeat TCB was done. The most probable explanation for the false-negative cases in the current study could be sampling error, slide misinterpretation or incorrect size of the needle used. Experts prefer to use larger needles in the evaluation of breast masses because of the propensity of these lesions to develop peripheral desmoplastic reactions. Furthermore, larger bore needles are able to contain larger volumes of sample tissue.18 However, at our institute; we use a narrow bore 18G biopsy needle to minimize mass displacement during the biopsy. For this reason, the probability of obtaining higher quality, more intact cores, and higher DA is decreased. Moreover, tumor cell displacement can occur, even in core biopsies.19
Conclusion
The diagnosis of benign changes or a normal breast by TCB would reassure the patient about the absence of malignancy and these cases can be followed-up in outpatient clinics. Patients in the current study who had benign breast lesions diagnosed accurately by TCB were saved a lot of expenses and did not undergo unnecessary surgical procedures that would have been an additional burden on the healthcare system. The use of TCB also lessens the propensity of complicated surgical procedures and minimizes patient stress. In patients with malignant lesions, in addition to having diagnostic significance, TCB also provides adequate tissue for the evaluation of molecular markers which have extreme therapeutic value. Therefore, we propose that TCB is an accurate alternative to FNAC for the diagnosis of breast lesions.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Saudi Cancer Registry Report 1999-2005. Saudi Cancer Registry, Saudi Oncology Society. www.oncology.org.sa.
2. Verenhitach BD, Elias S, Patrocínio AC, Nazário AC, Waizberg A. Evaluation of the clinical efficacy of minimally invasive procedures for breast cancer screening at a teaching hospital. J Clin Pathol 2011 Oct;64(10):858-861.
3. Mendoza P, Lacambra M, Tan PH, Tse GM. Fine needle aspiration cytology of the breast: the non-malignant categories. Patholog Res Int 2011; 2011:547580.
4. Berner A, Sauer T. Fine-needle aspiration cytology of the breast. Ultrastruct Pathol 2011 Aug;35(4):162-167.
5. Kurita T, Tsuchiya SI, Watarai Y, Yamamoto Y, Harada O, Yanagihara K, et al. Roles of fine-needle aspiration and core needle biopsy in the diagnosis of breast cancer. Breast Cancer 2012 Jan;19(1):23-29.
6. Devilee P, Tavassoli FA. (2003). World Health Organization: Tumours of the Breast and Female Genital Organs. Oxford [Oxfordshire]: Oxford University Press.
7. Sensitivity, specificity, positive predictive value and negative predictive value - Parkland Formula. www.josephsunny.com.
8. Hashemzadeh SH, Kumar PV, Malekpour N, Hashemi Z, Fattahi F, Malekpour F. Diagnostic accuracy of fine needle aspiration cytology: comparison of results in Tabriz Imam Khomeini Hospital and Shiraz University of Medical Sciences. Iranian J Cancer Prev 2009;2(3):133-136.
9. Yip CH, Jayaram G, Alhady SF. The experience with fine needle aspiration cytology in the management of palpable breast lumps in the University Hospital Kuala Lumpur. Me J Malaysia 2000; 55:363-7.
10. Bdour M, Hourani S, Mefleh W, Shabatat A, Karadsheh S, Nawaiseh O, et al. Comparison between fine needle aspiration cytology and tru-cut biopsy in the diagnosis of breast cancer. J Surg Pak 2008;13(1):19-21.
11. Yong WS, Chia KH, Poh WT, Wong CY. A comparison of trucut biopsy with fine needle aspiration cytology in the diagnosis of breast cancer. Singapore Med J 1999 Sep;40(9):587-589.
12. Lacambra MD, Lam CC, Mendoza P, Chan SK, Yu AM, Tsang JY, et al. Biopsy sampling of breast lesions: comparison of core needle- and vacuum-assisted breast biopsies. Breast Cancer Res Treat 2012 Apr;132(3):917-923.
13. Ahmed ME, Ahmad I, Akhtar S. Ultrasound guided fine needle aspiration cytology versus core biopsy in the preoperative assessment of non-palpable breast lesions. J Ayub Med Coll Abbottabad 2010 Apr-Jun;22(2):138-142.
14. Brunner AH, Sagmeister T, Kremer J, Riss P, Brustmann H. The accuracy of frozen section analysis in ultrasound-guided core needle biopsy of breast lesions. BMC Cancer 2009; 24:9:341.
15. Kulkarni D, Irvine T, Reyes RJ. The use of core biopsy imprint cytology in the ‘one-stop’ breast clinic. Eur J Surg Oncol 2009 Oct;35(10):1037-1040.
16. Homesh NA, Issa MA, El-Sofiani HA. The diagnostic accuracy of fine needle aspiration cytology versus core needle biopsy for palpable breast lump(s). Saudi Med J 2005 Jan;26(1):42-46.
17. Gukas ID, Nwana EJ, Ihezue CH, Momoh JT, Obekpa PO. Tru-cut biopsy of palpable breast lesions: a practical option for pre-operative diagnosis in developing countries. Cent Afr J Med 2000 May;46(5):127-130.
18. Estanislao JA, Deveza AD, Turingan HZ, Leyson MC, Go H, Yu AA, et al. Effect of needle gauge on yield and accuracy of aspiration biopsy in palpable breast masses. omsurg_research.tripod.com/needleguage_breast02.htm
19. Wong TE, Hisham AN. Core needle biopsy of palpable breast lump: the influence of needle size. Med J Malaysia 2003 Aug;58(3):399-404.
|
|