Over the past two decades, the prevalence of differentiated thyroid cancer (DTC), particularly papillary thyroid carcinoma, has increased worldwide.1 To some extent, this rising trend can be explained by the improved detection of cases following the development of more sensitive medical surveillance and diagnostic tools, as well as an increase in prognosis and survival among DTC patients; however, it is possible that this trend also reflects a true increase in incidence.2,3
Despite the recent increase in disease prevalence, the DTC-related mortality rate remains stable.3 Overall, the prognosis of this disease is favorable, with five-year survival rates reported to be as high as 95.8%.4 However, tumor persistence or recurrence was observed in 15–40% of DTC patients.5 Various factors are associated with poor outcomes, including older age, histological tumor subtype, invasion of the angiovascular structures and extension of the tumor to the extrathyroidal structures, regional lymph nodes, or distant regions.6 Understanding the relationship between prognostic factors and disease-free survival (DFS) is a key element in improving patient care, reducing the burden of treatment on the healthcare system, and tailoring long-term surveillance strategies.
In Oman, the prevalence of DTC is rising, with previous research showing that the number of cases increased by a factor of 5.5 between 2006 and 2016.7 However, to the best of our knowledge, no local studies have yet been conducted with regards to prognosis and prognostic factors in Oman. As such, this study aimed to assess the prognosis and prognostic factors of DTC among Omani patients attending a tertiary care center. These findings will ideally aid decision-makers and healthcare practitioners in establishing risk-based management guidelines.
Methods
This retrospective observational study was conducted between January 2006 and June 2016 at the National Diabetes and Endocrine Center (NDEC), a government tertiary care center in Oman, which provides care for diabetes and endocrine patients referred from secondary health centers across the country. All Omani patients aged 18 years and above who were diagnosed with DTC and followed up at the NDEC thyroid oncology clinic during this period were included in the study. Outcome data were tracked for each patient until their last follow-up visit, allowing for the calculation of outcome status three, five, and 10 years after diagnosis. Patient with incomplete information regarding disease status during the follow-up period were excluded from the study.
Data concerning the patients’ demographic and tumor characteristics were obtained from their electronic medical records. Information regarding age, body mass index, and thyroid function test (TFT) status was collected at the time of the initial diagnosis. Gender, tumor characteristics, lymph node status, distant metastases, biochemical profile (e.g., thyroglobulin (Tg), thyroglobulin antibody (TgAb), and thyroid-stimulating hormone (TSH) levels), and imaging results were recorded at subsequent follow-up visits. Data concerning tumor type and TNM status (primary tumor (T), regional lymph node (N), and distant metastasis (M) stage) were retrieved from histopathology reports.
All tumors were classified according to the 7th edition of the revised American Thyroid Association (ATA) TNM staging system.8 Following thyroidectomy, all patients underwent initial risk stratification according to ATA guidelines. Those with pT1 or pT2 tumors at N0M0 stage without aggressive histology were categorized as low risk, while those with aggressive histology or pT3, or N1 tumors were categorized as intermediate risk. Patients with pT4 tumors or tumors at any T or N stage with M1 progression were considered high risk. Prognostication staging was performed as per the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.9
All patients underwent a total thyroidectomy with or without lymph node dissection. Subsequently, patients underwent immediate postoperative evaluation involving a thyroid cancer profile (i.e., the measurement of stimulated Tg, TgAb, and TSH levels) and ultrasound examination of the neck. In addition, until 2015, all patients received radioactive iodine (I131) therapy, with this modality reserved thereafter for selected cases following the publication of the revised ATA management guidelines.10 Overall, most of the study population received a total of 100–150 mCi of I131 in the first two to three months following surgery.
To assess response to therapy, patients again underwent screening and ultrasonography of the neck within the first 12 months of treatment. This was repeated at one, three, five, and 10 years, as well as at the final follow-up visit. For analysis, the time of diagnosis was considered to constitute the baseline for survival. Patients were grouped according to disease status, with patients considered to be disease-free in the absence of clinical, biochemical (i.e., unstimulated serum Tg levels of < 0.2 µg/L or stimulated Tg levels of < 1 µg/L in the absence of interfering TgAbs), and radiological evidence of disease recurrence or persistence. In contrast, active disease was defined by the presence of one or more of the following:1 unstimulated serum Tg levels of ≥ 0.2 µg/L or stimulated Tg levels of ≥1 µg/L;2 a rising or denovo appearance of TgAb;3 and abnormal findings on radioimaging. For patients with active disease, additional examinations were performed, including a whole-body I131 diagnostic scan and computed tomography +/- positron emission tomography. In such cases, additional treatments involving I131 therapy, surgery, or radiotherapy were carried out at the discretion of the treating clinician.
Descriptive results were expressed as percentages or means with standard deviations (SDs). Associations were tested using univariate or multivariate tests. For the univariate analysis, chi-square or independent t-tests were performed according to the nature of the variables. For the multivariate analysis, binary logistic regression was used to adjust for potential confounders. The level of statistical significance was set at p < 0.050. All variables with associations at p < 0.250 in the univariate logistic regression analysis were considered candidates for inclusion in the binary logistic regression model. Kaplan-Meier curves were plotted, and log-rank tests conducted to compare prognostic factors for DFS. Data were analyzed using the (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). This study was approved by the Research Ethics Committee of Royal Hospital.
Table 1: Demographic and tumor characteristics of Omani patients with differentiated thyroid cancer (N = 346).
|
Age, years (range) |
38.2 ± 11.9 (18–80) |
|
Gender |
|
|
Male |
55 (15.9) |
|
Female |
291 (84.1) |
|
BMI, kg/m2 (range) |
26.9 ± 5.43 (17–42) |
|
TFT status at diagnosis |
|
|
Euthyroid |
259 (74.9) |
|
Hyperthyroid |
68 (19.7) |
|
Hypothyroid |
19 (5.5) |
|
Characteristics |
n (%) |
|
Histological subtype |
|
|
Classic PTC |
272 (78.6) |
|
FVPTC |
32 (9.2) |
|
OVPTC |
10 (2.9) |
|
Aggressive PTC |
5 (1.4) |
|
Minimally-invasive FTC |
18 (5.2) |
|
Widely-invasive FTC |
3 (0.9) |
|
Hurthle cell cancer |
6 (1.7) |
|
Tumor size in cm |
|
|
≤ 1 |
128 (37.0) |
|
1–2 |
97 (28.0) |
|
2–4 |
103 (29.8) |
|
≥ 4 |
18 (5.2) |
|
TNM staging* |
|
|
pT1a/Nx–N0/M0 |
102 (29.5) |
|
pT1b/Nx–N0/M0 |
61 (17.6) |
|
pT2/Nx–N0/M0 |
50 (14.5) |
|
pT3/Nx–N0/M0 |
20 (5.8) |
|
pT1–pT3/N1a/M0 |
64 (18.5) |
|
pT1–pT3/N1b/M0 |
39 (11.3) |
|
pT4/any N/M0 |
1 (0.3) |
|
Any T/any N/M1 |
9 (2.6) |
|
Lymph node status |
|
|
Nx |
160 (46.2) |
|
N0 |
78 (22.5) |
|
N1a |
58 (16.8) |
|
N1b |
50 (14.5) |
|
Number of lymph node metastases |
|
0 |
234 (67.6) |
|
1–5 |
85 (24.6) |
|
5–10 |
15 (4.3) |
|
≥ 10 |
12 (3.5) |
|
Presence of distant metastases |
|
Yes |
9 (2.6) |
|
No |
337 (97.4) |
|
Extrathyroidal extension |
|
|
Yes |
33 (9.5) |
|
No |
313 (90.5) |
|
Angiovascular invasion |
|
|
Yes |
30 (8.7) |
|
No |
316 (91.3) |
|
Tumor focality |
|
|
Unifocal |
204 (59.0) |
|
Multifocal |
142 (41.0) |
|
ATA risk category |
|
|
Low |
211 (61.0) |
|
Intermediate |
125 (36.1) |
|
High |
10 (2.9) |
|
AJCC prognostic stage |
|
|
I |
302 (87.3) |
|
II |
17 (4.9) |
|
III |
17 (4.9) |
|
IVA |
5 (1.4) |
|
IVB |
1 (0.3) |
SD: standard deviation; BMI: body mass index; TFT: thyroid function test; PTC: papillary carcinoma; FV: follicular variant; OV: oncocytic variant; FTC: follicular thyroid carcinoma; TNM: tumor, nodes, and metastases; ATA: American Thyroid Association; AJCC: American Joint Committee on Cancer. *According to the 7th edition of the revised American Thyroid Association TNM staging system.8
Table 2: Predictors of prognosis among Omani patients with differentiated thyroid cancer (N = 346).
|
Gender |
|
|
0.320 |
- |
|
Male |
87.3 |
12.7 |
|
|
|
Female |
81.8 |
18.2 |
|
|
|
Age, years |
|
|
0.600 |
- |
|
< 45 |
82.1 |
17.9 |
|
|
|
> 45 |
84.7 |
15.3 |
|
|
|
Duration of follow-up in months |
68.75 ± 30.74 |
67.75 ± 29.3 |
0.820 |
- |
|
TFT status at diagnosis |
|
|
0.290 |
- |
|
Euthyroid |
82.2 |
17.8 |
|
|
|
Hyperthyroid |
83.8 |
16.2 |
|
|
|
Hypothyroid |
84.2 |
15.8 |
|
|
|
Histological subtype |
|
|
0.010 |
0.900 |
|
Classic PTC |
79.8 |
20.2 |
|
|
|
FVPTC |
96.9 |
3.1 |
|
|
|
OVPTC |
100 |
0.0 |
|
|
|
Aggressive PTC |
80.0 |
20.0 |
|
|
|
Widely-invasive FTC |
33.3 |
66.7 |
|
|
|
Minimally-invasive FTC |
94.4 |
5.6 |
|
|
|
Hurthle cell cancer |
100 |
0.0 |
|
|
|
Tumor size in cm |
|
|
0.060 |
0.240 |
|
≤ 1 |
89.1 |
10.9 |
|
|
|
1–2 |
79.4 |
20.6 |
|
|
|
2–4 |
76.7 |
23.3 |
|
|
|
≥ 4 |
88.9 |
11.1 |
|
|
|
Lymph node status |
|
|
< 0.001 |
0.380 |
|
Nx |
92.5 |
7.5 |
|
|
|
N0 |
88.5 |
11.5 |
|
|
|
N1a |
74.1 |
25.9 |
|
|
|
N1b |
52.0 |
48.0 |
|
|
|
Number of lymph node metastases |
|
|
< 0.001 |
0.017 |
|
0 |
91.9 |
8.1 |
|
|
|
1–5 |
72.0 |
28.0 |
|
|
|
5–10 |
53.3 |
46.7 |
|
|
|
≥ 10 |
25.0 |
75.0 |
|
|
|
Distant metastasis status |
|
|
< 0.001 |
1.000 |
|
M0 |
84.3 |
15.7 |
|
|
|
M1 |
22.2 |
77.8 |
|
|
|
TNM stage* |
|
|
< 0.001 |
0.037 |
|
pT1a/Nx–N0/M0 |
33.1 |
12.9 |
|
|
|
pT1b/Nx–N0/M0 |
19.0 |
11.3 |
|
|
|
pT2/Nx–N0/M0 |
16.2 |
6.5 |
|
|
|
pT3/Nx–N0/M0 |
6.3 |
3.2 |
|
|
|
pT1–pT3/N1a/M0 |
15.8 |
30.6 |
|
|
|
pT1–pT3/N1b/M0 |
8.8 |
22.6 |
|
|
|
pT4/any N/M0 |
0.0 |
1.6 |
|
|
|
Any T/any N/M1 |
0.7 |
11.3 |
|
|
|
AJCC stage |
|
|
< 0.001 |
0.540 |
|
I |
85.1 |
14.9 |
|
|
|
II |
70.6 |
29.4 |
|
|
|
III |
82.4 |
17.6 |
|
|
|
IVA |
40.0 |
60.0 |
|
|
|
IVB |
0.0 |
100 |
|
|
|
IVC |
25.0 |
75.0 |
|
|
|
ATA risk category |
|
|
< 0.001 |
0.079 |
|
Low |
91.5 |
8.5 |
|
|
|
Intermediate |
72.8 |
27.2 |
|
|
|
High |
20.0 |
80.0 |
|
|
|
Tumor focality |
|
|
0.490 |
- |
|
Unifocal |
83.8 |
16.2 |
|
|
|
Multifocal |
81.0 |
19.0 |
|
|
|
Extrathyroidal extension |
|
|
< 0.001 |
0.180 |
|
No |
85.6 |
14.4 |
|
|
|
Yes |
54.5 |
45.5 |
|
|
|
Angiovascular invasion |
|
|
< 0.001 |
0.016 |
|
No |
86.1 |
14.9 |
|
|
SD: standard deviation; TFT: thyroid function test; PTC: papillary carcinoma; FV: follicular variant; OV: oncocytic variant; FTC: follicular thyroid carcinoma; TNM: tumor, nodes, and metastases; AJCC: American Joint Committee on Cancer; ATA: American Thyroid Association.
*According to the 7th edition of the revised American Thyroid Association TNM staging system.8
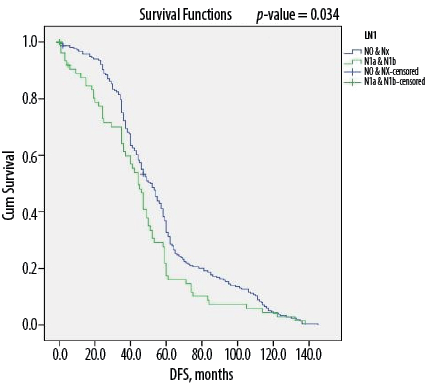
Figure 1: Kaplan-Meier survival curve showing disease-free survival (DFS) according to lymph node status among Omani patients with differentiated thyroid cancer (N = 346).
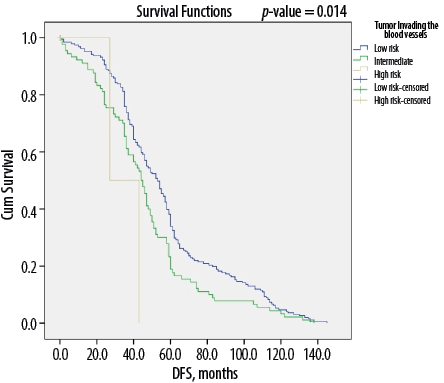
Figure 2: Kaplan-Meier survival curve showing disease-free survival (DFS) according to angiovascular invasion among Omani patients with differentiated thyroid cancer (N = 346).
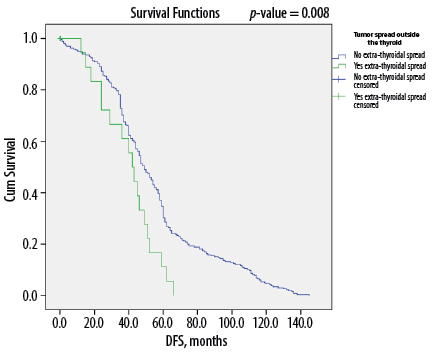
Figure 3: Kaplan-Meier survival curve showing disease-free survival (DFS) according to extrathyroidal extension among Omani patients with differentiated thyroid cancer (N = 346).
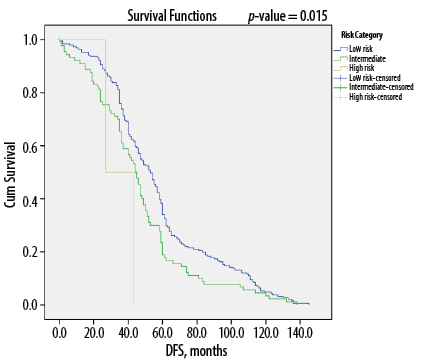
Figure 4: Kaplan-Meier survival curves showing disease-free survival (DFS) according to American Thyroid Association risk category among Omani patients with differentiated thyroid cancer (N = 346).
Results
Overall, 84.1% of patients were female. The mean age at diagnosis was 38.2±11.9 years old (range: 18–80 years), 90.2% of the sample were below 45 years of age. At their initial diagnosis, 74.9% were euthyroid, 19.7% were hyperthyroid, and 5.5% were hypothyroid [Table 1]. The mean duration of follow-up was 68.6±30.5 months (median: 60 months; range: 30–157 months).
In terms of histological subtype, 92.2% of cases were papillary tumors, with 78.6% representing classic papillary carcinoma. In contrast, only 1.7% and 0.9% were Hurthle cell cancer (HCC) and widely-invasive follicular thyroid carcinoma, respectively. The majority of patients (65.0%) had tumors < 2 cm in size, while 29.8% had 2–4 cm-sized tumors (pT2), and only 5.2% had tumors of > 4 cm (pT3). Extrathyroidal extension (ETE) was noted in 9.5% of cases, while angiovascular invasion was seen in 8.7%. A total of 142 patients (41.0%) had multifocal tumors. In addition, in 28.9% of cases, the tumor had metastasized to the cervical lymph nodes, although only 3.5% had 10 or more metastatic lymph nodes.
Overall, 61.6% of cases had localized disease (TNM stage pT1–pT2/N0/M0), while 35.8% demonstrated extension to regional lymph nodes or adjacent structures (pT3–pT4/N1a–N1b). Only 2.6% of tumors had spread to distant regions, such as the lungs and bone. Most cases (61.0%) were categorized as lower risk according to the ATA classification, while 2.9% were in the high-risk category. Upon initial prognostic staging, the majority of patients (87.3%) were in stage I, with only 1.2% in stage IVC. In terms of treatment, 80.0% received a single dose of I131 therapy, while 4.3% did not receive I131 treatment following surgery.
At the time of their last follow-up visit, 82.7% of the study population was disease-free compared to 32.7% at the initial follow-up visit. This represents a sharp increase in the percentage of patients who attained disease-free status over the study period.
As illustrated in Table 2, there was no significant association between disease-free status at the last follow-up visit with gender, age, duration of follow-up, or TFT status at diagnosis. However, in terms of tumor characteristics, approximately 80% of cases of papillary tumors (both classic and other variants) and 100% of cases of HCC were disease-free at their final follow-up visit, compared to only 33.3% of those with widely-invasive follicular thyroid cancer (p < 0.050). In addition, 89.1% and 88.9% of patients with tumors of ≤1 cm or ≥ 4 cm, respectively, were disease-free at last follow-up (p < 0.050).
Regarding lymph node status, 92.5% and 88.5% of patients with Nx and N0 status were disease-free at their final follow-up visit compared to those with N1a or N1b status (74.1% and 52.0%, respectively; p < 0.050). In addition, the majority of patients without ETE (85.6%), angiovascular invasion (86.1%), and distant metastasis (84.3%) were disease-free (p < 0.050). However, 75.0% of patients with AJCC stage IVC disease and 75.0% of those with ≥ 10 metastatic lymph nodes demonstrated disease recurrence or persistence (p < 0.050). In terms of ATA risk category, 91.5% of low-risk and 72.8% of intermediate-risk cases were disease-free at last follow-up, compared to 20.0% of those in the high-risk category (p < 0.050).
Overall, DTC patients without lymph node metastasis (i.e., N0 status) had a median of 56 months of DFS compared to 45 months for those with metastasis to the lymph nodes (p < 0.050) [Figure 1]. Similarly, median DFS was 54 months for cases without ETE and angiovascular invasion, compared to only 38 months in cases with extension into these structures (p < 0.050) [Figures 2 and 3]. Finally, as depicted in Figure 4, patients categorized as ATA low risk were disease-free for a longer period (57 months) compared to those in other risk categories (46 months and 35 months, respectively, for intermediate and high risk) (p < 0.050).
Discussion
This retrospective, observational cohort study evaluated the prognosis of 346 Omani patients with DTC and identified prognostic factors for DFS. In this study, the rate of DFS—defined as survival at last follow-up visit without evidence of persistent or recurrent disease—was 82.7% after a median of 60 months of follow-up. Various factors known to be related to the prognosis of patients with DTC were analyzed. Significant predictors of DFS included TNM staging indicating that the tumors were limited to the thyroid gland (i.e., T1–T2/N0/M0) without invasion to the surrounding structures or angiovascular system (p < 0.001). In contrast, metastasis to cervical lymph nodes or distant regions, ETE, and angiovascular invasion were strongly associated with persistent disease (p < 0.001). Moreover, as would be expected, longer DFS was observed for patients categorized as low-risk according to the ATA guidelines.8
A retrospective study of 231 South African patients with DTC, reported a similar recurrence-free survival rate (83%) over a 10-year follow-up period.11 However, limited research is available regarding the long-term outcome of DTC patients in the Gulf region. A study of 600 DTC patients from the King Faisal Specialist Hospital and Research Centre in Saudi Arabia observed a 53.3% DFS rate over a median of 7.6 years follow-up.12 However, low DFS in this study could be attributed to the comparatively high rate of patients with locally advanced disease compared to the present study (42.2% vs. 9.5%).12
Conflicting reports exist regarding the association between age and gender with disease outcomes in DTC patients. Hajj Boutros et al,13 noted that age at presentation (i.e., patients < 45 years old) was an important predictor of DFS. Similarly, Jonklaas et al,14 reported that DTC prognosis was better among females < 45 years of age compared to their age-matched male counterparts. In contrast, women diagnosed when they were > 55 years demonstrated disease-specific survival patterns indistinguishable from older men. In the aforementioned Saudi study, both the male gender and being ≥ 45 years of age were associated with poor disease outcomes.12 However, our study failed to determine a significant association between DFS with age (p = 0.600) or gender (p = 0.320). This might be explained by the fact that the disease was confined to the thyroid (without extension to the regional lymph nodes, extrathyroidal region, or vascular structures) for most patients, resulting in similar prognoses regardless of gender or age group. Although Robertson et al,11 observed higher DTC-related mortality rates among patients > 45 years, neither age nor gender was found to affect recurrence rate. A retrospective study from Argentina also reported similar results.15 However, age has been incorporated in to the AJCC prognostication staging for patients with DTC; the age cut-off has been raised from 45 to 55 years for poor prognosis of DTC group in the recent edition.9
Consistent with other studies, we failed to show an impact of obesity on the disease prognosis.16 In the present study, no significant association was observed between hyperthyroidism and disease outcome (p = 0.290). Previous research has revealed similar findings.17 While Mekraksakit et al,18 noted a high rate of tumor multifocality and distant metastasis among patients with Grave’s disease, disease outcomes for these patients did not differ compared to those with euthyroid DTC. However, conflicting results have been reported by other researchers.19
Widely-invasive follicular cancer is known to be associated with poor disease prognosis.20 Indeed, only a quarter of patients with widely-invasive follicular cancer in our study were disease-free at their last assessment. This is likely due to the presence of distant metastasis at initial presentation. However, consensus regarding the prognosis of HCC, a variant of follicular cancer, is unclear. Oluic et al,21 reported a favorable prognosis for patients with HCC, with 5-, 10-, and 20-year DFS rates of 91.1%, 86.2%, and 68.5%, respectively. In another study, Sugino et al,22 concluded that HCC does not have a poor prognosis, as only 5.5% of patients with HCC developed distant metastasis compared to 21.9% with follicular thyroid cancer. Similarly, all patients with HCC in our study were disease-free at their last follow-up visit. On the other hand, other researchers have demonstrated poor outcomes for this patient group.23 This may be explained by the fact that HCC patients in our study were comparatively younger, with disease more likely to be limited to the thyroid gland.
In our study, a significant number of patients with tumors < 1 cm and > 4 cm were disease-free at their last follow-up visit (p = 0.060). In contrast, a retrospective review of 2323 patients with DTC at Texas University in the USA showed lower rates of DFS among patients with large tumors ( > 4 cm) compared to those with small tumors.24 The previously mentioned Italian study and a meta-analysis from China also showed a strong association between recurrence/persistence of DTC and large tumor size.20,25 In these other studies, patients with large-sized tumors had higher rates of vascular invasion, lymph node, and distant metastasis, whereas only 5.2% of patients in our study had large-sized tumors, with most free from extensive disease; these differences might explain this variation in results.
The influence of tumor mutifocality on the prognosis of DTC patients is debatable. Some investigators have noted higher rates of non-remission and lower rates of DFS in multifocal DTC.26 However, in our study, although half of the patients had multifocal disease, most were found to be disease-free at last follow-up (p = 0.490). Comparable findings have been reported in Saudi Arabia and Italy.12,20
Overall, DTC has a high propensity for spreading to the regional lymph nodes, particularly when it comes to the papillary subtypes.11,12,27 At the time of the initial surgery, microscopic lymph node metastasis is expected in up to 80% of patients with papillary thyroid cancer, although the clinical impact of this type of micrometastasis is not significant.28 It was seen that persistent disease after initial surgery is both due to unidentified nodal disease pre-operatively or incomplete removal of the involved metastatic lymph nodes during surgery.20 The absence of lymph node metastasis (N0 status) was an independent prognostic variable for DFS (p < 0.001), with patients experiencing significantly longer disease-free periods compared to those with nodal metastatic cancer (p = 0.034). Similar conclusions have been reported by Guo et al,29 in a systematic review and meta-analysis, as well as by other researchers in more recent original studies.11,12
Another important finding of the present study was that DFS decreases significantly as the number of involved lymph nodes increases (p < 0.001). In a retrospective review of 115 papillary thyroid cancer patients, Lee et al,30 noted a high recurrence rate among patients with greater numbers of metastatic cervical lymph nodes. Other research similarly suggests that the higher the number of involved lymph nodes to the number of nodes studied (i.e., the L:N ratio), the greater the chances of disease recurrence.31 Furthermore, in this study, persistent disease was more often associated with metastasis to the lateral group of cervical lymph nodes (N1b status) compared to the central group, a finding consistent with those of other studies.32,33 These pieces of evidence stipulate a thorough preoperative assessment using appropriate imaging to evaluate the neck for nodal metastases to decide whether simultaneous lymphadenectomy is necessary. Miller et al34 suggested that prophylactic central neck dissection be considered for large and locally advanced tumors. This could lower the chance of persistent or recurrent disease, reducing the necessity of additional treatment, particularly re-operation following the initial surgery and I131 therapy.
Histological evidence of tumor cells within the lumen or walls of the tumoral vessels is defined as vascular invasion and is cause for the patient to be categorized as intermediate risk.10 As in other reports, vascular invasion was another independent variable for poor prognosis in our study (p = 0.001). Most patients with vascular involvement have N1b disease, with one in every five reported to have distant metastasis, thereby conferring a poorer prognosis.13,35 A retrospective study conducted by Falvo et al,36 indicated a high rate of lymph node involvement (20.5% vs. 3.8%) and distant metastasis (12.8% vs. 1.66%) in DTC patients with angiovascular invasion compared to those without invasion.
Recent studies have shown that microscopic tumor extension holds limited prognostic significance regarding recurrence-free survival, disease-specific survival, and persistent disease.37,38 However, in our study, ETE, whether micro- or macroscopic, was significantly lower among disease-free patients (p < 0.001). Park et al,39 reported identical results among 381 patients with DTC, with significantly lower five-year recurrence-free survival among patients with microscopic ETE than those without ETE (92.1% vs. 99.3%).
In light of such findings, most thyroid cancer guidelines for DTC recommend an initial and ongoing risk-adapted approach to management.10,40,41 This would allow for more accurate prognostication and the tailoring of therapy and follow-up strategies on an individual basis. Vaisman et al,42 observed recurrence rates of 13%, 36%, and 68% for DTC patients in low-, intermediate-, and high-risk groups, respectively. As with the findings of the present study, poor DFS was noted for patients in the high-risk group (p < 0.001).
The strengths of the present study include its relatively large sample size and long follow-up period. However, the authors acknowledge that this study is limited by the fact that it is retrospective in nature and confined to a single center. Nevertheless, as the NDEC is a tertiary endocrine center receiving patients from all regions of Oman, the results of this study likely reflect the prognosis of DTC patients throughout the country.
Conclusion
Most DTC patients in the present study were disease-free at their last follow-up visit, indicating a favorable prognosis. However, treatment should be tailored on an individual basis with regard to specific risk factors. Implementation of this type of risk-based therapeutic approach would help minimize the overall impact of the growing number of DTC cases in Oman.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212.
- 2. Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid 2014 Mar;24(3):472-479.
- 3. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014 Apr;140(4):317-322.
- 4. Borges AK, Ferreira JD, Koifman S, Koifman RJ. Differentiated thyroid carcinoma: a 5-years survival study at a referral hospital in Brazil. Rev Saude Publica 2019 Nov;53:106.
- 5. Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 2013 Dec;154(6):1436-1446.
- 6. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 2015 Jul;33(21):2370-2375.
- 7. Pambinezhuth F, Al Busaidi N, Al Musalhi H. Epidemiology of thyroid cancer in Oman. Annals of Endocrinology and Metabolism 2017;1:11-17.
- 8. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009 Nov;19(11):1167-1214.
- 9. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67(2):93-99.
- 10. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid modules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016 Jan;26(1):1-133.
- 11. Robertson B, Parker M, Shepherd L, Panieri E, Cairncross L, Malherbe F, et al. Nodal disease predicts recurrence whereas other traditional factors affect survival in a cohort of South African patients with differentiated thyroid carcinoma. Cancers Head Neck 2018 Nov;3(1):10.
- 12. Alzahrani AS, Alomar H, Alzahrani N. Thyroid cancer in Saudi Arabia: a histopathological and outcome study. Int J Endocrinol 2017;2017:8423147.
- 13. Hajj Boutros R, Arabi A, Shoucair M, Abbas J, Salti I. Disease free survival of well differentiated thyroid cancer: 20 years’ experience at a tertiary care center in Lebanon. Int Arch Med 2018;11:2545.
- 14. Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, et al; National Thyroid Cancer Treatment Cooperative Study Group. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 2012 Jun;97(6):E878-E887.
- 15. Pitoia F, Jerkovich F, Smulever A, Brenta G, Bueno F, Cross G. Should age at diagnosis be included as an additional variable in the risk of recurrence classification system in patients with differentiated thyroid cancer. Eur Thyroid J 2017 Jul;6(3):160-166.
- 16. Gąsior-Perczak D, Pałyga I, Szymonek M, Kowalik A, Walczyk A, Kopczyński J, et al. The impact of BMI on clinical progress, response to treatment, and disease course in patients with differentiated thyroid cancer. PLoS One 2018 Oct;13(10):e0204668.
- 17. MacFarland SP, Bauer AJ, Adzick NS, Surrey LF, Noyes J, Kazahaya K, et al. Disease burden and outcome in children and young adults with concurrent Graves disease and differentiated thyroid carcinoma. J Clin Endocrinol Metab 2018 Aug;103(8):2918-2925.
- 18. Mekraksakit P, Rattanawong P, Karnchanasorn R, Kanitsoraphan C, Leelaviwat N, Poonsombudlert K, et al. Prognosis of differentiated thyroid carcinoma in patients with Graves disease: a systematic review and meta-analysis. Endocr Pract 2019 Dec;25(12):1323-1337.
- 19. Medas F, Erdas E, Canu GL, Longheu A, Pisano G, Tuveri M, et al. Does hyperthyroidism worsen prognosis of thyroid carcinoma? A retrospective analysis on 2820 consecutive thyroidectomies. J Otolaryngol Head Neck Surg 2018 Jan;47(1):6.
- 20. Sapuppo G, Tavarelli M, Belfiore A, Vigneri R, Pellegriti G. Time to separate persistent from recurrent differentiated thyroid cancer: different conditions with different outcomes. J Clin Endocrinol Metab 2019 Feb;104(2):258-265.
- 21. Oluic B, Paunovic I, Loncar Z, Djukic V, Diklic A, Jovanovic M, et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: a single center experience. BMC Cancer 2017 May;17(1):371.
- 22. Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, et al. Does Hürthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann Surg Oncol 2013 Sep;20(9):2944-2950.
- 23. Ahmadi S, Stang M, Jiang XS, Sosa JA. Hürthle cell carcinoma: current perspectives. Onco Targets Ther 2016 Nov;9:6873-6884.
- 24. Tam S, Amit M, Boonsripitayanon M, Busaidy NL, Cabanillas ME, Waguespack SG, et al. Effect of tumor size and minimal extrathyroidal extension in patients with differentiated thyroid cancer. Thyroid 2018 Aug;28(8):982-990.
- 25. Choi JB, Lee SG, Kim MJ, Kim TH, Ban EJ, Lee CR, et al. Oncologic outcomes in patients with 1-cm to 4-cm differentiated thyroid carcinoma according to extent of thyroidectomy. Head Neck 2019 Jan;41(1):56-63.
- 26. Ng SC, Kuo SF, Chen ST, Hsueh C, Huang BY, Lin JD. Therapeutic outcomes of patients with multifocal papillary thyroid microcarcinomas and larger tumors. Int J Endocrinol 2017;2017:4208178.
- 27. Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol 2016 Apr;12(7):981-994.
- 28. Chang YW, Kim HS, Jung SP, Kim HY, Lee JB, Bae JW, et al. Significance of micrometastases in the calculation of the lymph node ratio for papillary thyroid cancer. Ann Surg Treat Res 2017 Mar;92(3):117-122.
- 29. Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol 2014 Aug;7(9):5393-5403.
- 30. Lee J, Song Y, Soh EY. Prognostic significance of the number of metastatic lymph nodes to stratify the risk of recurrence. World J Surg 2014 Apr;38(4):858-862.
- 31. Pyo JS, Sohn JH, Chang K. Prognostic role of metastatic lymph node ratio in papillary thyroid carcinoma. J Pathol Transl Med 2018 Sep;52(5):331-338.
- 32. Sapuppo G, Palermo F, Russo M, Tavarelli M, Masucci R, Squatrito S, et al. Latero-cervical lymph node metastases (N1b) represent an additional risk factor for papillary thyroid cancer outcome. J Endocrinol Invest 2017 Dec;40(12):1355-1363.
- 33. Liu YQ, Li H, Liu JR, Lin YS. Unfavorable responses to radioiodine therapy in N1b papillary thyroid cancer: a propensity score matching study. Endocr Pract 2019 Dec;25(12):1286-1294.
- 34. Miller JE, Al-Attar NC, Brown OH, Shaughness GG, Rosculet NP, Avram AM, et al. Location and causation of residual lymph node metastasis after surgical treatment of regionally advanced differentiated thyroid cancer. Thyroid 2018 May;28(5):593-600.
- 35. Gardner RE, Tuttle RM, Burman KD, Haddady S, Truman C, Sparling YH, et al. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2000 Mar;126(3):309-312.
- 36. Falvo L, Catania A, D’Andrea V, Marzullo A, Giustiniani MC, De Antoni E. Prognostic importance of histologic vascular invasion in papillary thyroid carcinoma. Ann Surg 2005 Apr;241(4):640-646.
- 37. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014 Feb;24(2):241-244.
- 38. Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM, et al. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery 2011 Dec;150(6):1242-1249.
- 39. Park JS, Chang JW, Liu L, Jung SN, Koo BS. Clinical implications of microscopic extrathyroidal extension in patients with papillary thyroid carcinoma. Oral Oncol 2017 Sep;72:183-187.
- 40. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30(12):1856-1883.
- 41. Pitoia F, Ward L, Wohllk N, Friguglietti C, Tomimori E, Gauna A, et al. Recommendations of the Latin American thyroid society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol 2009 Oct;53(7):884-887.
- 42. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 2012;77(1):132-138.