Although chylothorax is the most common form of pleural effusion in neonates,1 chylous effusion following umbilical venous catheter (UVC) insertion is an extremely rare entity. It can result in significant respiratory compromise if not promptly diagnosed and treated. In a neonate with respiratory distress following UVC insertion, pleural effusion should be considered as a possibility. Although few cases have been reported in the western literature, no similar cases are available from the Middle East.
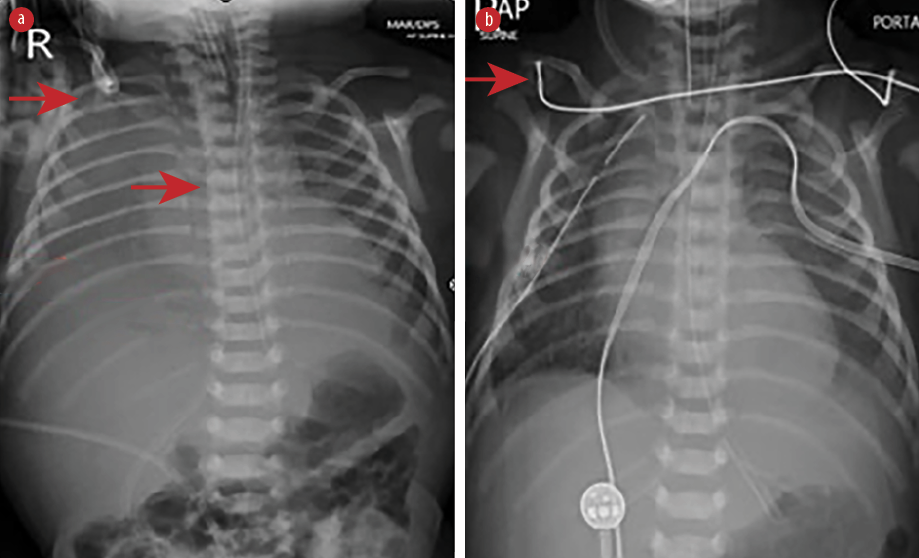
Figure 1: Chest radiograph shows (a) right-sided pleural effusion with the catheter tip in the correct position (red arrows) and (b) intercostal drain on the right side (red arrows).
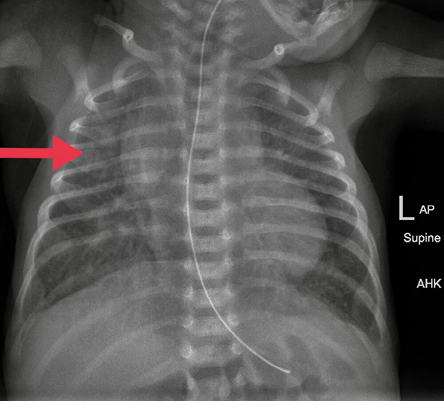
Figure 2: Chest radiograph after right thoracentesis. The catheter was removed. Lung fields were clear.
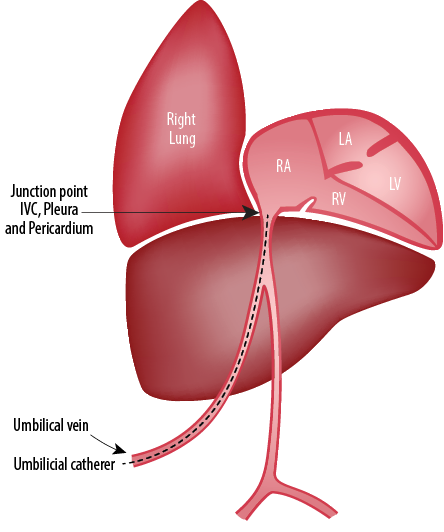
Figure 3: Site where the intrathoracic extra pericardial portion of the inferior vena cava (IVC) comes in contact with the right pleura.3
Case report
A 2650 gram male baby born at 37 weeks of gestation to a G4 PO A3 mother by normal vaginal delivery with normal Apgar scores was referred to us at 22 hours of life on mechanical ventilation. The antenatal period was complicated by gestational diabetes mellitus. The prenatal echocardiogram (ECHO) was suggestive of a hypoplastic left heart. Shortly after birth, the neonate was taken to the neonatal intensive care unit and placed on non-invasive respiratory support due to hypoxemia and bradypnea. Postnatal ECHO showed hypoplastic right heart with single ventricle physiology for which the baby was started on prostaglandin E1 infusion and intravenous fluids. UVC French five was inserted at the referring hospital and was fixed at 8.5 cm length at umbilical stump level. The position was confirmed by chest X-ray.
Physical examination after admission to our unit revealed decreased air entry on the right side with respiratory distress. Chest radiograph showed complete haziness of the right lung field. An ultrasound of the chest repeated at the same time showed massive pleural effusion on the right side with UVC tip in situ [Figure 1]. UVC tip was in an appropriate position at the junction of the inferior vena cava (IVC) and right atrium during the ECHO study for cardiac disease evaluation. Fifty milliliters of serous fluid was drained [Figure 1a], and an intercostal drain (ICD) was inserted [Figure 1b] to prevent recollection as the baby was on high ventilator parameters and the cause for pleural effusion was not known during ICD insertion. In anticipation of the baby’s needs to be kept nil by mouth for a longer duration of time, the baby was started on total parenteral nutrition (TPN) with lipids and proteins on day two as two septic screens done 18 hours apart were negative. The ICD became milky with a similar appearance to TPN after starting TPN, and 160 mL of milky fluid was drained. Pleural fluid analysis revealed 112 cell counts with lymphocytic predominance (70%), 200/mm3 red blood cells, no organism seen on Gram stain, lactate dehydrogenase of 45 U/L, triglyceride of 980 mg/dL, and glucose of 780 mg/dL. Pleural fluid to serum lactate dehydrogenase ratio was < 0.6. Pleural fluid culture was sterile. TPN fluid analysis was sent, and it also showed a similar composition as pleural effusion. We suspected the UVC had migrated to the pleural cavity, as no blood was aspirated through the UVC. The UVC was removed, and a percutaneously inserted central catheter line was inserted and TPN continued. The neonate remained asymptomatic after UVC removal and drain decreased drastically to < 10 mL over the next 24 hours and became serous again. ICD was clamped and then removed later as there was no recollection. There was no recurrence of pleural effusion as evidenced by chest X-ray [Figure 2] 24 hours after removing the ICD. The baby was extubated after ICD removal and subsequently taken for cardiac surgery on day five of life. There was no associated vascular anomaly found during cardiac surgery, and the baby was discharged postoperatively from the cardiac intensive care unit.
Discussion
Chronology of events: drainage of milky fluid from the pleural cavities, no recurrence of the pleural collection after removal of the umbilical venous line, high triglycerides in the pleural fluid support an association of umbilical vein catheter with pleural effusion in the infant on parenteral nutrition. The most common complication of UVC is malposition. On the other hand, pleural or pericardial effusions rarely occur.2 Two possible explanations for umbilical line-induced pleural effusions are line migration and hyperosmolar endothelial damage, which may be the reason in our case. The anatomical relation of IVC and pleuropericardial cavities [Figure 3] can explain the reasons for pleuropericardial collections.3 Migration of catheter tip may occur because of movement of head and extremities and flushing of the UVC. In two reported cases, UVCs were in the proper position, but both were complicated by pleural and pericardial effusions.4,5 Pabalan et al,3 reported right-sided pleural effusion in a 28 week preterm infant following umbilical venous catheterization. The complication developed nearly 40 hours after the line insertion. Chest X-ray revealed normal tip position of the catheter. Drainage and catheter removal helped in the complete resolution of the pleural effusion. Another case reported umbilical venous line related pleural and pericardial effusion in a 34 week preterm infant.6 In their case, the newborn was started on parenteral nutrition on day two of life, developed pleural effusion five days and pericardial effusion eight days after insertion of the UVC. The symptoms cleared only after removal of the catheter on day 13 of life. The catheter position and tip were always appropriate, and there was no evidence of line migration. Kumar et al,7 also have reported bilateral pleural effusion following UVC, which resolved after removal of the catheter. Unal et al,8 also have reported a similar case of pleural and pericardial effusion as a complication of properly placed UVC. The verification of catheter tip is important both at placement and during follow-up. Even though we excluded the possibility of malposition of the catheter tip by X-ray and ECHO, the presented case showed that proper positioning does not avoid complications. It is remarkable that isolated pericardial effusions were mostly reported due to malpositioned UVCs, whereas pleural effusions were mostly reported with properly placed UVCs.9
Conclusion
The UVCs have considerable benefits in neonates though complications might arise in up to 20% of patients. Although malposition is the most common complication, pleural effusion occurs rarely. This can present with worsening respiratory distress in an apparently normal baby. Hence in a neonate developing respiratory distress following UVCs, clinicians should always keep in mind the possibility of pleural effusion. In any neonate with pleural effusion with indwelling UVCs, pleural effusion secondary to UVC should be suspected, even if the position of UVC is appropriate. UVC should be aspirated to see if any backflow and laboratory analysis of pleural fluid and infusate should be done. UVC should be removed promptly in such cases, which in all the cases will act as a therapeutic intervention.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the patient.
references
- 1. Tutor JD. Chylothorax in infants and children. Pediatrics 2014 Apr;133(4):722-733.
- 2. Mutlu M, Aslan Y, Kul S, Yılmaz G. Umbilical venous catheter complications in newborns: a 6-year single-center experience. J Matern Fetal Neonatal Med 2016 Sep;29(17):2817-2822.
- 3. Pabalan MJ, Wynn RJ, Reynolds AM, Ryan RM, Youssfi M, Manja V, et al. Pleural effusion with parenteral nutrition solution: an unusual complication of an “appropriately” placed umbilical venous catheter. Am J Perinatol 2007 Nov;24(10):581-585.
- 4. Alabsi S. Neonatal cardiac tamponade and pleural effusion resolved with chest tube placement. Neonatal Netw 2010 Nov-Dec;29(6):347-351.
- 5. Garg G, Mandhan G, Sidana P. Bilateral pleural effusion, cardiogenic shock, renal failure, and generalized anasarca: a dreaded iatrogenic complication of umbilical venous catheterization. Ann Pediatr Cardiol 2016 Jan-Apr;9(1):108-109.
- 6. Hong EJ, Lee KA, Bae H, Kim MJ, Han HS. Umbilical venous line related pleural and pericardial effusion causing cardiac tamponade in premature neonate. Korean J Pediatr 2006;49(6):686-689.
- 8. Kumar N, Murki S. Bilateral pleural effusion complicating umbilical venous catheterization. Indian Pediatr 2013 Dec;50(12):1157-1158.
- 9. Unal S, Arifoglu I, Celik IH, Yilmaz O, Bas AY, Demirel N. Pleural and pericardiac effusion as a complication of properly placed umbilical venous catheter. J Neonatal Surg 2017 Apr;6(2):34.
- 10. Abdellatif M, Ahmed A, Alsenaidi K. Cardiac tamponade due to umbilical venous catheter in the newborn. BMJ Case Rep 2012 Jul;2012:bcr-2012-6160.