|
Abstract
Objectives: To study the characteristics of cardiovascular risk factors in regard to age (before and after 60) and gender. Many reports refer to the higher prevalence of cardiovascular risk factors among the younger type 2 diabetics in comparison with the older population.
Methods: The study included 462 randomly recruited type 2 diabetic subjects (above and below 60 years) attending Al-Zahrawi Private Hospital in Mosul City-Iraq, during the period from June to August 2011. They were analyzed in regard to age, duration of diabetes, smoking, socio economic status, anthropometric indices, blood pressure, fasting plasma glucose, glycated hemoglobin A1c and serum lipids. Data were analyzed using chi-square and unpaired Z test.
Results: Duration of diabetes, diastolic blood pressure, glycated hemoglobin A1c, fasting plasma glucose, serum lipids, number of hypercholesterolemic patients, number of patients having unfavorable total cholesterol/HDL ratio (≥5) and positive family history of coronary heart disease were all significantly higher in the younger diabetics. In addition, younger diabetic females were distinguished by a larger number of hypertensive patients, higher level of systolic blood pressure, higher means of body mass index, total cholesterol and LDL, and larger number of patients having low HDL-C (<1 mmol/L). The younger diabetic males were distinct bya larger number of smokers, number of smoked cigarettes/day, and longer duration of smoking. All parameters ranged between p<0.05 and p<0.005.
Conclusion: Cardiovascular risk factors were significantly higher among younger type 2 diabetics (<60 years), particularly females.
Keywords: Type 2 diabetes; HbA1c%; Fasting plasma glucose; Lipid profile; Smoking; Cardiovascular risk factors; Dyslipidemia; Age.
Introduction
Diabetes mellitus (DM) is a world-wide endemic with increasing prevalence in both developing and developed countries.1 It is well-established as one of the most serious risk factors for coronary heart disease (CHD),2 and a common cause of disability and death all over the world.3 Because of this, the Adult Treatment Panel III (ATP III) raises persons with DM without CHD to the risk level of CHD.4
Diabetes that affects all age groups is pathologically classified into two broad categories, type 1 DM (T1DM) and type 2 DM (T2DM). T1DM is characterized by beta-cell destruction leading to absolute insulin deficiency and is further classified into two subgroups: 1) T1DM-A that results from autoimmune destruction of beta cell, and 2) T1DM-B that lacks immunologic markers of an autoimmune destruction of beta cells; however, the affected subjects develop insulin deficiency by unknown mechanisms and are prone to ketosis. T2DM accounts for 90% to 95% of diabetes; it is a heterogeneous group of disorders characterized by insulin resistance, impaired insulin secretion and increased glucose production.5,6
Diabetes progressively increases with age. In 2005, the prevalence of DM in the United States was estimated to be 0.22% for persons <20 years of age and 9.6% in those >20 years of age. In individuals >60 years old, the prevalence of DM was 20.9%.5
Coronary heart disease that is attributed mainly to associated dyslipidemia, with the risk of endothelial dysfunction and chronic inflammation, is two to four times greater in patients with T2DM.5,7,8 Other factors such as obesity and hypertension, also play an important role in the macrovascular complications.5 These events correlate with glycemic control mainly fasting plasma glucose (FPG), postprandial plasma glucose levels, and glycated hemoglobin A1c (HbA1c%); however, tight glycemic control burdens patients with complex treatment programs, hypoglycemia, weight gain, costs, and offers uncertain benefits in return.9
Although both non-diabetic and diabetic men had higher cardiovascular risk factors than their female counterparts, the gender difference was smaller in the diabetic than in the non-diabetic populations.10,11 Other studies showed a more markedly increased cardiovascular risk factors in diabetic women compared with diabetic men.12-14 Among T2DM patients, there are reports referring to the higher prevalence of cardiovascular risk factors among the younger subgroup of T2DM in comparison with the older one.15,16 However, cardiovascular risk factors among the younger T2DM patients were not comprehensively studied in regard to gender. The aim of this study was to explore the general and gender characteristics of the younger (<60 years) and the older (≥60 years) T2DM subjects.
Method
This study was conducted in Mosul City, Iraq, during the period of June to August 2011, on 462 type 2 diabetic subjects (218 females and 244 males) who were attending the Outpatient Clinic at Al-Zahrawi Private Hospital. The age of the subjects ranged between 37-75 years with a mean age (SD) of 52.5 (6.4) years. The subjects were segregated into two groups: a younger age group (age: <60 years; n=315) and an older group (age: ³60 years; n=147). All the subjects were using conventional therapy for diabetes in addition to dieting. After obtaining their written consent, the subjects were examined for weight, height, and for their blood pressure (BP). The information pertaining to age, gender, duration of diabetes, cigarette smoking, family history of CHD, and socioeconomic status was recorded in special case records. Blood samples were gathered from the subjects to conduct a series of laboratory investigations using standard protocols for estimation of FPG, HbA1c%, total cholesterol, triglycerides (TG), low density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C).
Smoking status was determined by questionnaires, which were designed by interviewers according to WHO guidelines.17 According to the number of cigarettes smoked per day, the smokers were divided into three categories: 1-10, 11-20 and >20 cigarette/day. Furthermore, the smokers were divided into three groups according to the duration of smoking: 5 years, 6-10 years and >10 years.
The means of height and weight were used to calculate the body mass index (BMI).18 According to BMI, individuals were classified into normal weight (BMI: 18-24.99 kg/m2), overweight (BMI: 25-29.99 kg/m2), and obese (BMI: ≥30 kg/m2).19 Classification of dyslipidemia was based on ATP III categorization using lipoprotein thresholds of ≥1.7 mmol/L for TG, ³5.2 mmol/L for total cholesterol, >2.6 mmol/L for LDL-C and <1.0 mmol/L for HDL-C as cut-off limits for dyslipidemia.4 For total cholesterol/HDL-C ratio, a figure of ³5 was considered as a cut-off limit for dyslipidemia according to the recommendation of the British Hyperlipidemia Association.20
The presence of first-degree relatives who manifest CHD whether premature (in males <50 years and females <60 years),21 or not, and/or cerebrovascular diseases, and/or peripheral artery diseases was considered a positive familial history as well.
Diagnostic criteria for diabetes were used according to 2007 guidelines of the American Diabetes Association. A subject with a fasting plasma glucose of 126 mg/dL and above was considered to be diabetic.22 Glycemic control among diabetic subjects was based on the WHO recommendation and classified according to FPG (mmol/L) concentration into: good (FPG: <6.7), fair (FPG: 6.7-8.9), and bad (FPG: ≥8.9).23 Depending on HbA1c% measurement, the glycemic control was categorized as optimal (<6.8%), acceptable (6.8% to 7.6%), and poor control (³7.6%).24
Regarding BP, it was measured from the right arm after the individual had rested for 5 minutes in a sitting position. Hypertension was considered to be present by a self-report or if the systolic BP is >140 mmHg or the diastolic BP is >90 mmHg.25
In the morning (8 am - 9 am) after an overnight fast, venous blood samples (10 mL each) were collected from each subject during single outpatient visit. Plasma glucose was measured by oxidase peroxide method.26 Glycated hemoglobin was measured in whole blood by ion exchange resin quantitative colorimetric determination.27
Determination of total cholesterol, HDL-C, and TG was performed using enzymatic methods,28 and LDL-C was calculated.29 VLDL-C(mmol/L) was estimated by the formula: VLDL-C (mmol/L) = TG × 0.455.29
Statistical analyses included Z test with values quoted as mean (SD) for within gender comparisons between the two age groups (<60 years old vs. ≥60 years old). Chi-square test was used for comparison of the percentages. The p-value at <0.05 was considered statistically significant.
Results
The mean (SD) age of those who were <60 years (n=315) was 51.6 (5.0) years and the mean (SD) age of subjects who were ≥60 years (n=147) was 64.6 (4.8) years. The male-to-female ratio among the younger age group was 167/148 (1.2/1) and the male-to-female ratio of the older was 77/70 (1.1/1). (Table 1)
The younger diabetic subjects were found to have a significantly longer duration of disease in comparison with the older subjects. The younger diabetic subjects mean (SD) of diabetes was 7.4 (4.0) years vs. the older: 4.4 (3.1) years (p<0.005). (Table 2)
The younger diabetic subjects, particularly the males, showed a larger number of smokers (73.1%) in comparison with the older subjects (67%; p<0.05). Also the younger diabetic subjects generally indulged on larger number of cigarettes per day than the older patients (Table 2). Smoking levels of 10-20 and >20 cigarette/day were seen more in the younger than the older patients (p<0.005), while the lower level of smoking (<10/day) was significantly more prone among the older subjects.
In regard to gender, the younger males were heavier smokers than the older males (p value ranged between <0.05-<0.005), but there was no significant difference between the number of smoked cigarettes in regard to females. (Table 2)
The duration of smoking was longer among the younger diabetic subjects, particularly the males (p-value ranged between <0.05- <0.005). While the younger females showed no significant difference in the duration of smoking in comparison to the older females.
Table 1: Anthropometric measures and blood pressure of the two groups.
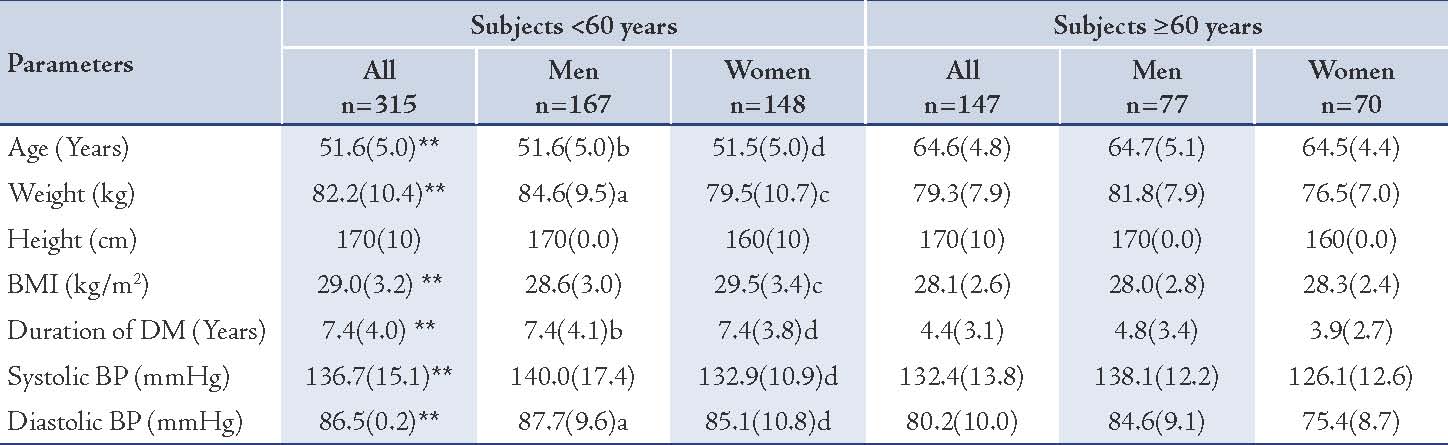
Values represented as mean (SD); p values were represented by stars as * and ** and by letters (a, b, cand d) as follows: * p<0.05, ** p<0.005 for all subjects: <60 years vs. ≥60 years, a= p<0.05, b= p<0.005 for men: <60 years vs. ≥60 years, c= p<0.05, d= p<0.005 for women: <60 years vs. ≥60 years.
Table 2: Frequency distribution of the study sample by demographic variables.
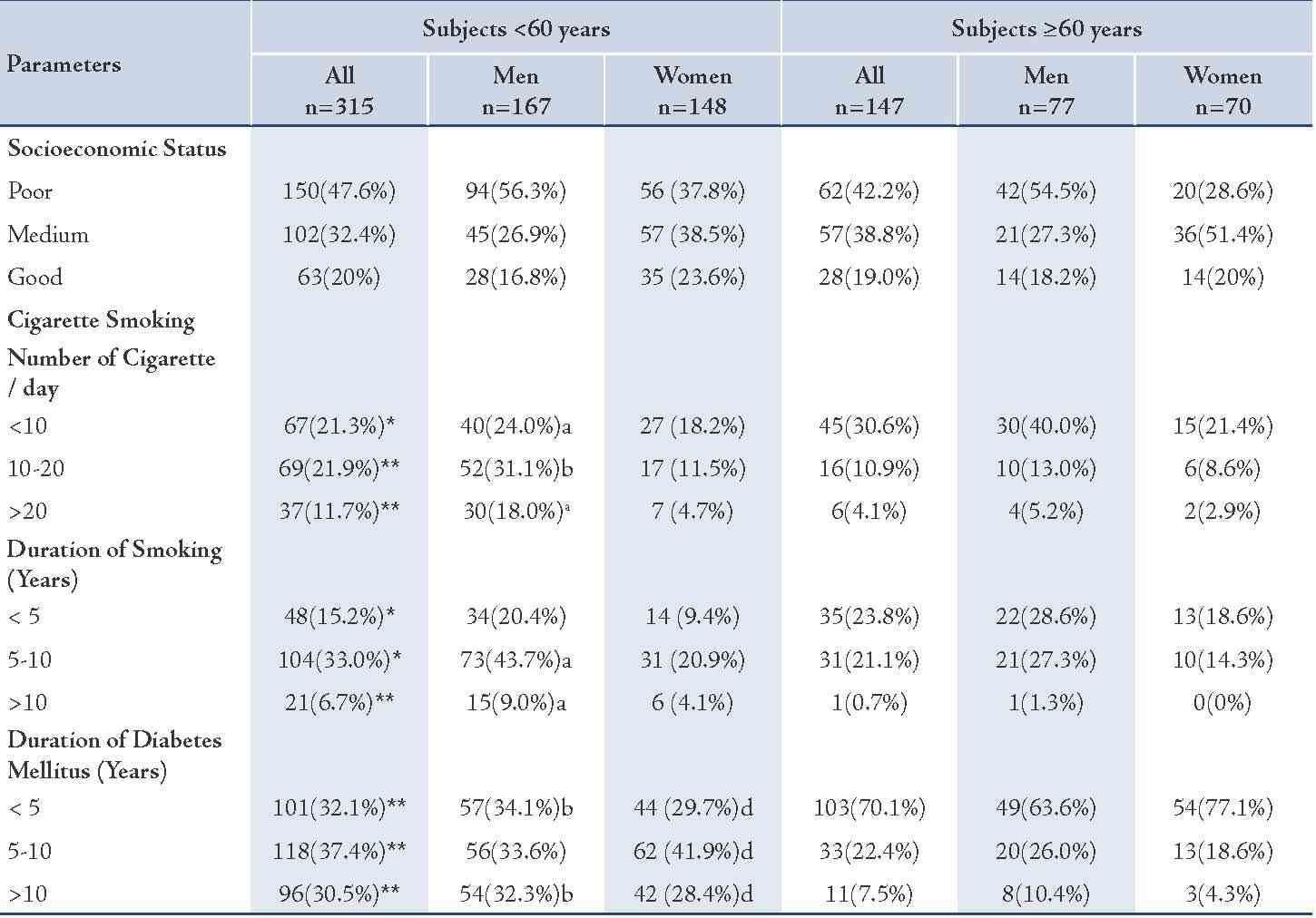
Values represented as number of subjects (n) and percentage (%), p values were assigned by star * and **and by letters (a, b, c and d) as follows: *= p<0.05; **= p<0.005 for all subjects <60 years vs. ≥60 years; a= p<0.05, b= p<0.005 for men <60 years vs. ≥60 years; c= p<0.05, d= p<0.005 for women <60 years vs. ≥60 years.
The younger females showed a higher number of hypertensive subjects among them (48; 32.4%) compared to the older females (7; 10%), p<0.005, (Table 3). While the number of younger male subjects having systolic hypertension, in general, was marginally higher than among the older group (NS), the number of subjects having systemic hypertension was significantly higher among the younger females in comparison to the older ones: 46 (31.1%) vs. 7 (10%), p<0.005. (Table 3)
The number of subjects having diastolic hypertension was significantly higher among the younger subjects in general and in the younger females but was only slightly higher in the younger males in comparison with the older males (NS). (Table 3)
Table 3: Assessment of the prevalence of CHD risk factors in the two groups.
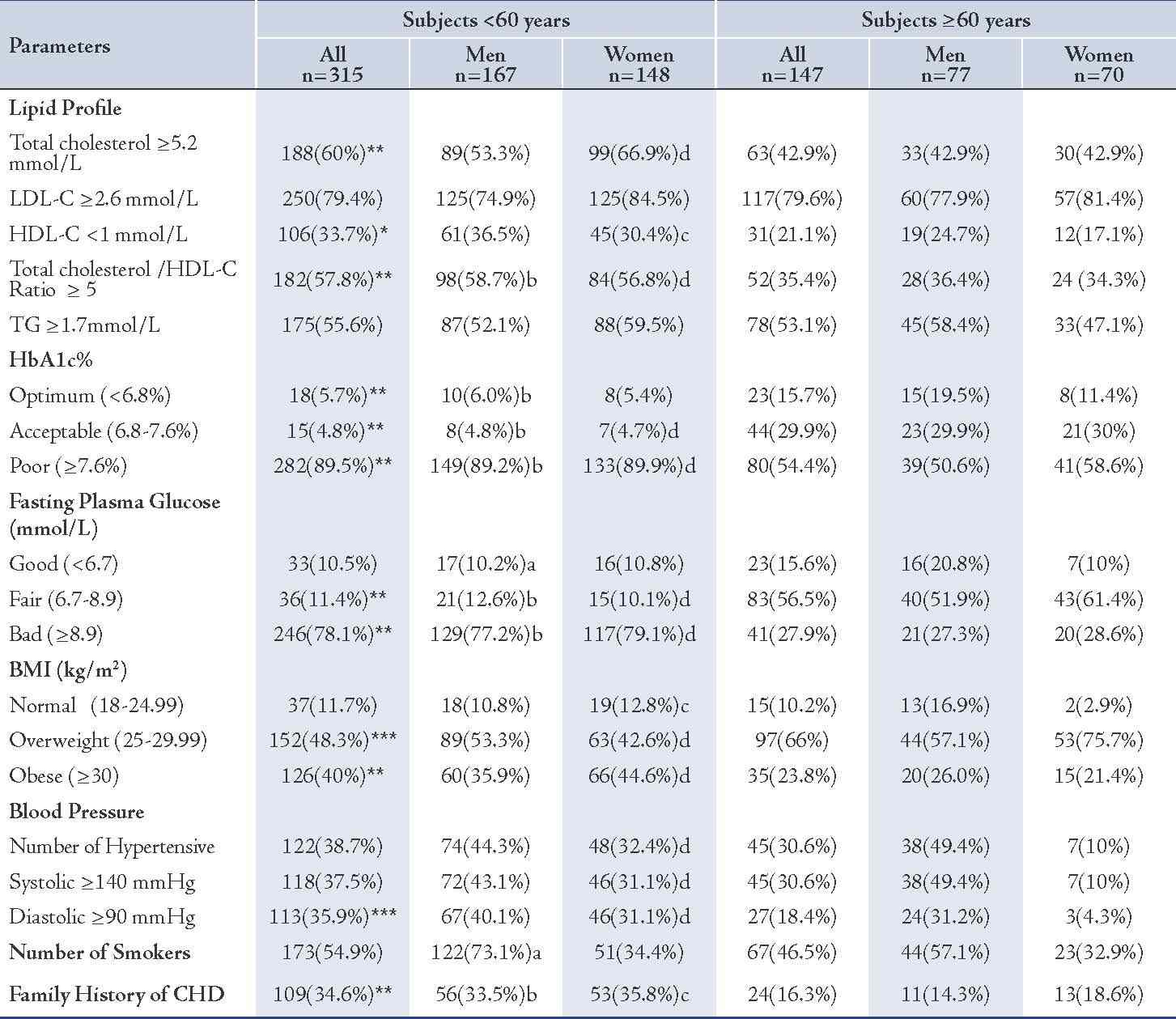
Values represented as number of subjects (n) and percentage (%). p values were represented by stars as * and ** and by letters (a, b, c and d) as follows: * p<0.05, ** p<0.005, for all subjects: <60 years vs. ≥60 years; a= p<0.05, b= p<0.005 for men: <60 years vs. ≥60 years; c= p<0.05, d= p<0.005 for women: <60 years vs. ≥60 years.
The mean (SD) of systolic BP was significantly higher in the younger diabetic subjects; in general and in females, in comparison with the older subjects (136.7 [15.1] mmHg vs. 132.4 [13.8] mmHg [p<0.005] and 132.9 [10.9] mmHg vs. 126.1 [12.6] mmHg [p<0.005], respectively). In the younger males, it was just marginally higher in comparison with the older males (NS), (Table 1). The mean (SD) diastolic BP was significantly higher in both the younger males and females (87.7 [9.6] mmHg vs. 80.2 [10.0] mmHg [p<0.05] and 85.1 [10.8] mmHg vs. 75.4 [8.7] mmHg [p<0.005] respectively). (Table 1)
The BMI values of the enrolled subjects ranged between 20- 37.3 kg/m2. The BMI of the younger diabetic subjects, as well as in females was generally significantly higher than in older patients, but the younger males showed only marginal (NS) increment of BMI in comparison with the older group. (Table 1)
The younger diabetic subjects, in general, males and females showed a significantly higher mean (SD) of HbA1c% in comparison with the older subjects (p<0.005), (Table 1). Moreover, the mean (SD) of FPG was significantly higher among the younger diabetic subjects, in general, males and females, in comparison with the older subjects (p<0.005). (Table 4)
The prevalence of hypercholesterolemic subjects who have a total cholesterol ≥5.2 mmol/L was higher in the younger diabetic subjects in general (60% vs. 42.9%; p<0.005) and in females (66.9% vs. 42.9%; p<0.005), in comparison with the older diabetics. In the males, the difference was marginal (NS). (Table 3)
The mean (SD) level of total cholesterol was significantly higher in the younger subjects, in general (5.5 [1.2] mmol/L vs. 5.2 [0.9] mmol/L; p<0.05) and in females (5.6 [1.2] mmol/L vs. 5.3 [0.8] mmol/L; p<0.05), but marginally higher (NS) in the younger males. (Table 4)
Table 4: Selected biochemical parameters of the two groups.
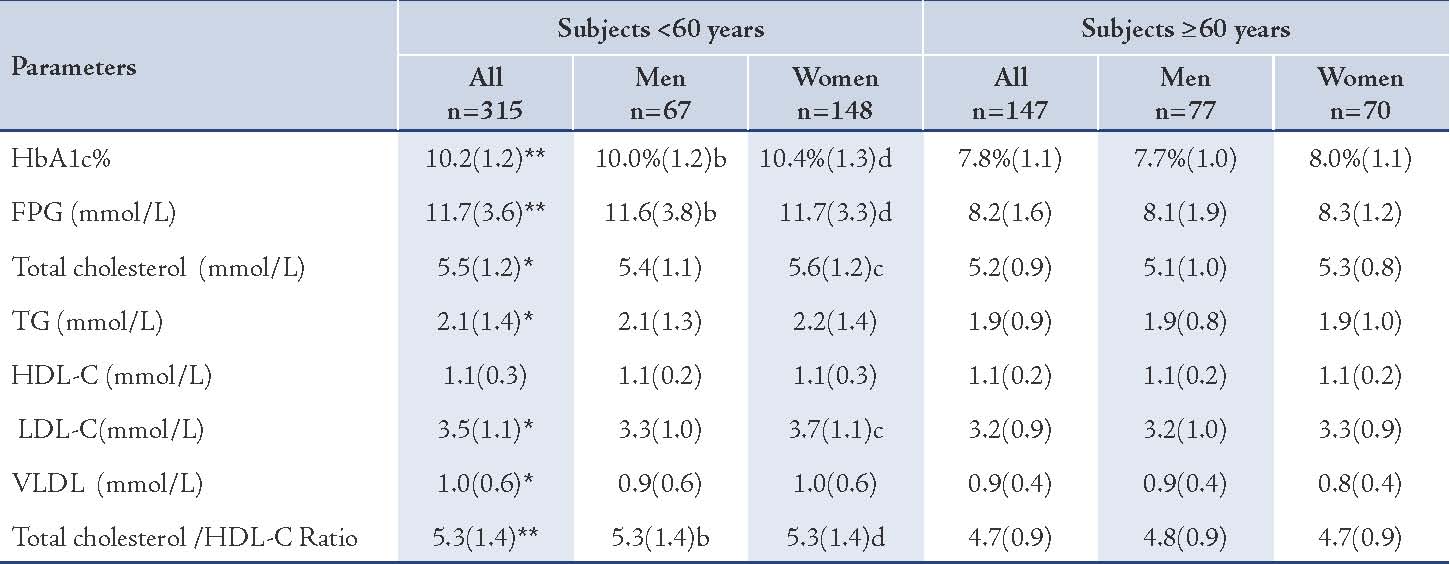
Values represented as mean (SD). p values were represented by stars as * and ** and by letters (a, b, c, and d) as follows: * p<0.05, ** p<0.005 for all subjects: <60 years vs. ≥60 years, a=p<0.05, b=p<0.005 for men: <60 years vs. ≥60 years, c=p<0.05, d=p<0.005 for women: <60 years vs. ≥60 years.
The difference in prevalence of excess levels of LDL-C was insignificant between the younger and the older subgroups. The mean (SD) level of LDL-C was significantly higher in the younger patients, as well as females (3.7 [1.1] mmol/L vs. 3.3 [0.9] mmol/L; p<0.05]), compared with the older diabetic subjects, but the males showed trivial excess (NS). (Table 4)
Low levels of HDL-C (<1 mmol/L) were significantly of higher prevalence among the younger diabetic subjects in general and in females, when compared with the older subjects (30.4% vs. 17.1%; p<0.005). While the mean (SD) concentration of HDL-C was nearly the same in the younger and the older subjects (NS). (Table 4)
The prevalence of diabetic subjects who have unfavorable total cholestreol/HDL-C ratio (≥5) was significantly higher among the younger subjects than the older groups (57.8% vs. 35.4%; p<0.005), (Table 3). The mean (SD) of total cholesterol/HDL-C ratio was generally significantly higher among the younger diabetic subjects and in both males and females, (Table 4). The total cholesterol/HDL-C ratio of the younger diabetic subjects in general was 5.3 (1.4) vs. 4.7 (0.9), p<0.005.
The prevalence hypertriglyceridemia (≥1.7mmol/L) among the younger subjects was significantly higher than the older, in general, but it was insignificantly different in regard to gender, (Table 3). The mean (SD) level of TG concentrations was significantly higher among the younger than the older subjects in general (2.1 [1.4] mmol/L vs. 1.9 [0.9] mmol/L, p<0.05) but insignificantly different between the males and females. (Table 4)
The mean (SD) concentration of VLDL in the younger group was generally higher than that of the older group (1.0 [0.6] mmol/L vs. 0.9 [0.4] mmol/L; p<0.05), (Table 4). However, the prevalence of positive family history of CHD was greater among the younger patients, in general and in both males and females, as opposed to the older subjects (34.6% vs. 16.3%; 33.5% vs. 16.3%; and 35.8% vs. 18.6%, respectively), p<0.005. (Table 3)
In general, the younger subjects, both males and females showed no significant difference in the level of socioeconomic status in comparison with the older subjects. (Table 2)
Discussion
Type 2 diabetes is a growing pandemic and is associated with rapid increase in its complications such as CHD.15,30 Aging was assumed to be an independent risk factor of CHD,31 but does it confer the same threat among diabetic population?
In this study, the characteristics of T2DM subjects, particularly the cardiovascular risk factors, were explored with regard to age and gender.
In the present study, most of the explored CHD risk factors appeared to condense among the younger diabetic subjects who were <60-year-old in comparison with the older subjects. The younger diabetic subjects in general, both the male and the female subgroups shared a significantly longer duration of diabetes, higher diastolic BP, higher HbA1c%, higher FPG, larger number of hypercholesterolemic subjects, larger number of unfavorable total cholesterol/HDL-C ratio (≥) and higher records of family history of CHD than in the older diabetic group. All these trends have adverse effect on the outcome of diabetes among the younger age group. Hypertension is a firm risk factor for atherosclerosis. Studies have shown that hypertension contributes significantly to the risk for atherosclerosis even with the added risks of high cholesterol and smoking.32,33 Elevated HbA1c% is an independent risk factor for CHD and stroke in diabetic subjects.16 Improving the glycemic control can markedly reduce the risk of cardiovascular events in diabetic subjects.34-36 Fasting plasma glucose and post challenge glucose concentrations are independent predictors of CHD risk as well.37
Although there is a considerable variability in classification into high and low risk subjects when using the total cholesterol concentration alone compared with compound risk indices like total cholesterol/HDL-C ratio or atherogenic index,38 HDL-C and total cholesterol/HDL-C ratios are important cardiovascular risk factors. Hence, treatment of familial hypercholesterolemia should focus not only on lowering total cholesterol and LDL-C levels but also on increasing HDL-C values for CHD prevention.39 In the UK Prospective Diabetes Study (UKPDS), the typical lipid pattern in the diabetic population compared with non-diabetics showed a pattern of hypertriglyceridemia, low HDL-C, relatively unaltered total cholesterol and increased LDL-C levels in females.40
What makes the younger group of diabetic population (<60 years) to have higher rates of cardiovascular risk factors in comparison with the older group? The most likely explanation is the higher prevalence of obesity among the younger subgroups in comparison with the older subgroups (BMI: 29.0 [3.2] vs. 28.1 [2.6]). The higher prevalence of obesity itself has contributed to the higher prevalence of cardiac risk factors in the female sector of the younger population themselves (BMI: 28.6 [3.0] for younger males vs. 29.5 [3.4] for the younger females). The younger diabetic females were distinguished by having a significantly higher mean (SD) BMI, a larger number of hypertensive subjects, a higher systolic BP, a higher total cholesterol and LDL-C means, and a larger number of subjects with low HDLC (<1 mmol/L) than the younger diabetic males.
There was no difference in the duration of diabetes in the younger males and females (7.4 [4.1] years for males vs. 7.4 [3.8] years for females); in spite of that, the females showed a higher prevalence of cardiovascular risk factors among them. This indicates that the effect of diabetes duration is not the factor that stands behind the acquisition of higher cardiovascular risk factor by the younger population in comparison with the older diabetic population who exhibited less cardiovascular risk factors. Obesity, particularly visceral or abdominal, is very common in T2DM.5 In accordance with other studies,16,36,41 the present study confirms that obesity is particularly high among the younger T2DM females rather than among the younger male diabetic subjects. Although waist circumference is a better indicator of health risks,42 obesity, whether phenomenologically manifested as visceral and its attendant metabolic syndrome, or as a subcutaneous, is firmly associated with increased cardiovascular risk.43,44 ATP III recognizes the cluster of visceral obesity, atherogenic LDL, low HDL-C, raised BP and insulin resistance, as a criteria for the diagnosis of metabolic syndrome.45
Thus, the significantly higher BMI detected in the younger females, frankly interprets the acquisition of the other adverse cardiovascular factors by them. Studies have disclosed that diabetes markedly increases the risk of cardiovascular complications in both males and females46; however, the diabetic females in general are more vulnerable to increased cardiovascular risk factors and mortality than diabetic males.47-50 The cause of the relatively higher risk of CHD in diabetic females is not completely understood but several explanations can be suggested. Adverse changes induced by T2DM in HDL-C, total cholesterol, TG, LDL-C particle size and BP have been found to be more pronounced in females than in males.47,49,51 The higher sum of CHD risk factors that, in this study were found to prevail among the younger diabetic subjects in general and the younger females in particular, presents another explanation for the higher risk of CHD among the diabetic females. Having a great exposure to cardiovascular risk, the subgroup of young diabetic females requires a great medical concern and comprehensive care provision to avoid the major cardiovascular events.
In addition to the cardiovascular factors that were found to be shared by all younger T2DM subjects in this study, the younger diabetic males showed more risky smoking trends than the older males. The number of smokers, the number of smoked cigarettes per day and the duration of smoking were significantly higher among the younger males than among the older males. Smoking is an independent risk factor for all-cause mortality, largely due to cardiovascular disease.52 Smoking, in addition to the other risks that were generally higher among the younger diabetics, exposes the younger male T2DM at a markedly increased risk of CHD as well.
All of the displayed CHD risk indices among the younger diabetics that pertain to dyslipidemia,53 hypertension,53,54 smoking habits,55 and obesity have a heritable propensity,56 hence the significantly higher recognition of family history of CHD among the younger diabetic males and females. Considering the heritable batch of cardiovascular risk factors that attend T2DM in the younger age group, it appears that early-onset T2DM, before the age of 60, is a familial disorder in comparison with the late-onset T2DM that exist above the age of 60. Seung et al. recorded that the metabolic syndrome and cardiovascular risk factors are more prevalent in familial T2DM than they were in non-familial T2DM.57 Similar trends were noted in MI that occurs in the younger age group. Positive family history of CHD, obesity and dyslipidemia were significantly higher in the younger-aged acute MI patients in comparison to the older-aged acute MI group.58
When the mean duration of T2DM in the present study is subtracted from the mean age of the younger and the older diabetic, the age of onset of diabetes in the two groups can be deduced. Thus the age of onset of diabetes in the younger group was about 44 years and the age of onset of diabetes in the older group was about 60. The number of subjects having a >10 years duration of diabetes were generally significantly higher among the younger age group, as well as in both males and females, than among the older age group. Both early and late onset diabetes are associated with the increased risk of major CHD events and mortality, but only the early onset diabetes (with >10 years duration) appears to be a CHD equivalent.59 As the young diabetics have their disease in the 5th decade (nearly at the age of 44), they become more vulnerable to the marked reduction in life spans that the diabetics usually sustain.
The prevailing overweight/obesity and the associated aberrancies in serum lipids and BP were generally more prominent among the younger diabetics and the younger females in particular, making them a distinct group of T2DM with regards to clinical features, comorbidities, targets of therapy, and prognosis. The older T2DM subjects with later age of onset have less severe comorbidities and CHD risk factors. The obesity in general and the metabolic syndrome in particular are expected to play a lesser role in the pathogenesis of late-onset T2DM and its evolution.
Being so, are we dealing with two distinct subgroups of T2DM that differ pathogenically, clinically and prognostically, perhaps T2DM-A before the age of 60 and T2DM-B thereafter?
Conclusion
Cardiovascular risk factors are significantly higher among younger type 2 diabetics (<60 years), particularly females, including dyslipidemia, smoking trends, hypertension, high body mass index, and in addition, the non-modifiable risk factor of the positive family history. This group of diabetics requires more stringent approach of therapy.
Acknowledgements
The authors reported no conflict of interest and no funding was received in this work.
References
1. Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol 2007 Feb;49(6):631-642.
2. Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med 2004 Jun;350(24):2438-2440.
3. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008 Jan;117(4):e25-e146.
4. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002 Dec;106(25):3143-3421.
5. Powers AC. Diabetes mellitus, In: Harrison's principles of internal medicine. Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, Loscalzo J. Eds. 17th edition. McGraw-Hill, New York. 2008: 2275-2279.
6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004 Jan;27(1)(Suppl 1):S5-S10.
7. Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundam Clin Pharmacol 2009 Dec;23(6):681-685.
8. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004 Mar;27(3):813-823.
9. Montori VM, Fernández-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med 2009 Jun;150(11):803-808.
10. Hyvärinen M, Tuomilehto J, Laatikainen T, Söderberg S, Eliasson M, Nilsson P, et al. The impact of diabetes on coronary heart disease differs from that on ischaemic stroke with regard to the gender. Cardiovasc Diabetol 2009;8:17.
11. Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002 Aug;162(15):1737-1745.
12. Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 2000 Jul;23(7):962-968.
13. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004 Sep;364(9438):937-952.
14. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006 Jan;332(7533):73-78.
15. Ezenwaka CE, Offiah NV. Differences in cardiovascular disease risk factors in elderly and younger patients with type 2 diabetes in the West Indies. Singapore Med J 2002 Oct;43(10):497-503.
16. Gunathilake S, Song S, Sridharan DJ, Fernando, Idris I. Cardiovascular and metabolic risk profiles in young and old patients with Type 2 diabetes. QJM; First published online guidelines for controlling and monitoring the tobacco epidemic. Geneva: July 30, 2010.
17. Guidelines for controlling and monitoring the tobacco epidemic. Geneva World Health Organization 1998.
18. Obesity—preventing and managing the global epidemic: report of a WHO consultation on obesity. Geneva, World Health Organization, 1998.
19. World Health Organization (WHO). Physical Status: the use and interpretation of anthropometry. Geneva: WHO, 1995. (Technical Report Series, 854).
20. Wood D, Durrington P, Poulter N. Joint British recommendations on prevention of coronary heart disease in clinical practice. Heart 1998;80(Suppl.2):1-29.
21. Bloomfield P, Bradbury A, Grubb NR, Newby DE. Cardiovascular diseases. In Davidson's Principles and Practice of Medicine. Boon NA, Colledge NRP and Walker BR Eds. 20th ed. Churchill Livingstone. Edinburgh 2006. 519-646.
22. American Diabetes Association. Standards of medical care in diabetes-2007. Diabetes Care 2007;1:S4-S5.
23. WHO-1993 EM/DIA. 3/E/G cited by Mansour AA. Type 2 diabetes mellitus: presentations, complication and treatment. Med J Basrah Univ 2002;20(1):41-47.
24. Stanbio. Glycohemoglobin procedure: principles, application and comparisons. East Houston Street, San Antonio, USA 2003; 1-20.
25. Awtry EH, Locscalo J. Coronary heart disease. In: Cecil Essential of Medicine. Capenter C; Griggs RC; and Locscalo J, Eds. 6th ed. Elsevier Saunders Inc. Philadelphia 2004: 87-173.
26. Sacks DB. Carbohydrates. In: Burtis CA, Ashood ER, Bruns DE, Eds. In Teitz Fundamentals of Clinical Chemistry. 6th ed. 2008: WB Saunders Philadelphia. 373-401.
27. Moore JC, Bown E, Outlaw MC, Jelfs R, Holman RR, Turner RC. Glycosylated haemoglobin: comparison of five different methods, including measurement on capillary blood samples. Ann Clin Biochem 1986 Jan;23(Pt 1):85-91.
28. Rifai N. Lipids, lipoproteins, aoplipoprotiens and other cardiovascular risk factors. In: Teitz Fundamentals of Clinical Chemistry. Burtis CA, Ashood ER and Bruns DE, Eds. 6th ed. WB Saunders. Philadelphia. 2008:402- 430.
29. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the ultracentrifuge. Clin Chem 1972;18:449-552.
30. Gholap N, Davies M, Patel K, Sattar N, Khunti K. Type 2 diabetes and cardiovascular disease in South Asians. Prim Care Diabetes 2011 Apr;5(1):45-56.
31. Zhan Y, Yuan L, Oettgen P. Alterations in transcriptional responses associated with vascular aging. J Inflamm (Lond) 2009;6:16.
32. Stamler J, Neaton JD, Wentworth DN. Blood pressure (systolic and diastolic) and risk of fatal coronary heart disease. Hypertension 1989 May;13(5)(Suppl):I2-I12.
33. Kannel WB, Neaton JD, Wentworth D, Thomas HE, Stamler J, Hulley SB, et al. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT. Multiple Risk Factor Intervention Trial. Am Heart J 1986 Oct;112(4):825-836.
34. Kawasumi M, Tanaka Y, Uchino H, Shimizu T, Tamura Y, Sato F, et al. Strict glycemic control ameliorates the increase of carotid IMT in patients with type 2 diabetes. Endocr J 2006 Feb;53(1):45-50.
35. Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J (Clin Res Ed) 1983 Sep;287(6396):867-870.
36. Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2006 Apr;29(4):877-882.
37. Chien KL, Hsu HC, Su TC, Chen MF, Lee YT, Hu FB. Fasting and postchallenge hyperglycemia and risk of cardiovascular disease in Chinese: the Chin-Shan Community Cardiovascular Cohort study. Am Heart J 2008 Nov;156(5):996-1002.
38. Berg JE, Høstmark AT. Cardiovascular risk determination: discrepancy between total cholesterol evaluation and two compound laboratory indices in Norway. J Epidemiol Community Health 1994 Aug;48(4):338-343.
39. Real JT, Chaves FJ, Martínez-Usó I, García-García AB, Ascaso JF, Carmena R. Importance of HDL cholesterol levels and the total/ HDL cholesterol ratio as a risk factor for coronary heart disease in molecularly defined heterozygous familial hypercholesterolaemia. Eur Heart J 2001 Mar;22(6):465-471.
40. U.K. Prospective Diabetes Study 27. Plasma lipids and lipoproteins at diagnosis of NIDDM by age and sex. Diabetes Care 1997 Nov;20(11):1683-1687.
41. Toner JM, Close CF, Ramsay LE. Factors related to treatment resistance in hypertension. Q J Med 1990 Nov;77(283):1195-1204.
42. Janssen I, Katzmarzyk PT, Ross RS. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004 Mar;79(3):379-384.
43. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weigHt. loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the council on nutrition, physical activity, and metabolism. Circulation 2006 Feb;113(6):898-918.
44. Ostlund RE Jr, Staten M, Kohrt WM, Schultz J, Malley M. The ratio of waist-to-hip circumference, plasma insulin level, and glucose intolerance as independent predictors of the HDL2 cholesterol level in older adults. N Engl J Med 1990 Jan;322(4):229-234.
45. Peter L. Prevention and treatment of atherosclerosis. In: Harrison's principles of internal medicine. Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser S, Jameson FL, Eds. 16th edition. McGraw-Hill, New York. 2005: 1432.
46. Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA 1999 Apr;281(14):1291-1297.
47. Howard BV, Cowan LD, Go O, Welty TK, Robbins DC, Lee ET. Adverse effects of diabetes on multiple cardiovascular disease risk factors in women. The Strong Heart Study. Diabetes Care 1998 Aug;21(8):1258-1265.
48. Petitti DB, Imperatore G, Palla SL, Daniels SR, Dolan LM, Kershnar AK, et al; SEARCH for Diabetes in Youth Study Group. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med 2007 Feb;161(2):159-165.
49. Walden CE, Knopp RH, Wahl PW, Beach KW, Strandness E Jr. Sex differences in the effect of diabetes mellitus on lipoprotein triglyceride and cholesterol concentrations. N Engl J Med 1984 Oct;311(15):953-959.
50. Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004 Dec;27(12):2898-2904.
51. Siegel RD, Cupples A, Schaefer EJ, Wilson PW. Lipoproteins, apolipoproteins, and low-density lipoprotein size among diabetics in the Framingham offspring study. Metabolism 1996 Oct;45(10):1267-1272.
52. Moy CS, LaPorte RE, Dorman JS, Songer TJ, Orchard TJ, Kuller LH, et al. Insulin-dependent diabetes mellitus mortality. The risk of cigarette smoking. Circulation 1990 Jul;82(1):37-43.
53. Park MH, Kim N, Lee JY, Park HY. Genetic loci associated with lipid concentrations and cardiovascular risk factors in the Korean population. J Med Genet 2011 Jan;48(1):10-15.
54. Luft FC. Twins in cardiovascular genetic research. Hypertension 2001 Feb;37(2 Part 2):350-356.
55. Batra V, Patkar AA, Berrettini WH, Weinstein SP, Leone FT. The genetic determinants of smoking. Chest 2003 May;123(5):1730-1739.
56. de Castro JM, Lilenfeld LR. Influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutrition 2005 Apr;21(4):446-455.
57. Jeong SU, Kang DG, Lee DH, Lee KW, Lim DM, Kim BJ, et al. Clinical characteristics of type 2 diabetes patients according to family history of diabetes. Korean Diabetes J 2010 Aug;34(4):222-228.
58. Chen JH, Huang HH, Yen DH, Wu YL, Wang LM, Lee CH. Different clinical presentations in chinese people with acute myocardial infarction in the emergency department. J Chin Med Assoc 2006 Nov;69(11):517-522.
59. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med 2011 Mar;171(5):404-410.
|