Perivascular epithelioid cell tumors (PEComas) are a rare group of neoplasms, includes renal angiomyolipoma (AML), lymphangioleiomyomatosis (LAM), and clear cell (sugar) tumor of the lung.1 These tumors are characterized by distinctive cells that show a close association with vascular structures and usually express melanocytic and smooth muscle markers.1
Epithelioid angiomyolipoma (EAML) is a variant of AML that has been described by Pea et al, in 1998.2 Unlike classical AML, which is composed of dysmorphic blood vessels, smooth muscle, and adipose tissue, EAML is predominantly composed of sheets of epithelioid cells that can have a high degree of cytoplasmic pleomorphism and atypia. In addition, EAMLs can have necrosis, which make it difficult to be differentiated from renal cell carcinoma.3–5 The imaging appearance of EAML has been less reported compared to its histopathological features.3 Previous reported cases in the literature have shown that EAML has minimal or no fat, and hence, the diagnosis can be challenging and difficult.3–5 Herein, we report a patient who had bilateral renal masses and the magnetic resonance imaging (MRI) findings were suggestive of EAML.
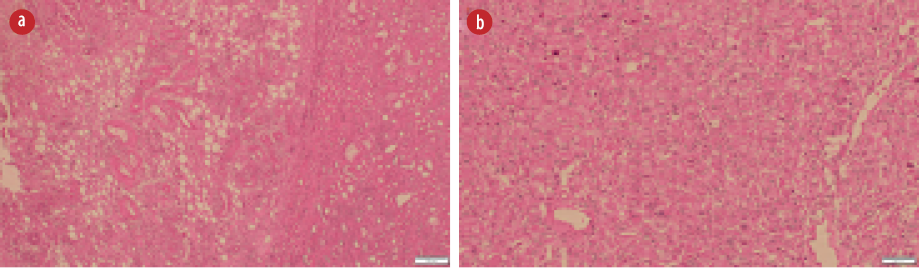
Figure 1: (a) Axial and (b) coronal T2-weighted images show bilateral hyperintense renal masses replacing renal parenchyma (right renal mass: green star, left renal mass: red star). (c) Axial T1 in-phase and (d) T1 out-phase show a large area of drop in signal intensity of right renal mass (green star). Small focal of signal drop (red arrow) seen in the left renal mass (red arrow).
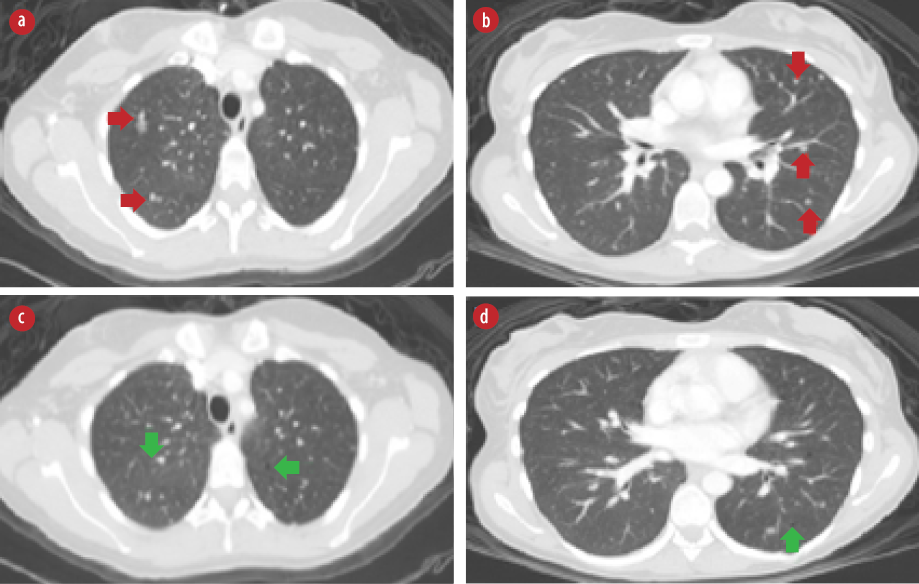
Figure 2: (a) Hematoxylin and eosin (H&E) stain showing many abnormally formed thick blood vessels, some with radiating smooth muscle fibers in the adjacent stroma, and adipose tissue component.
(b) H&E slide with sheets of large polygonal epithelioid cells with pleomorphic nuclei and plenty of eosinophilic cytoplasm.
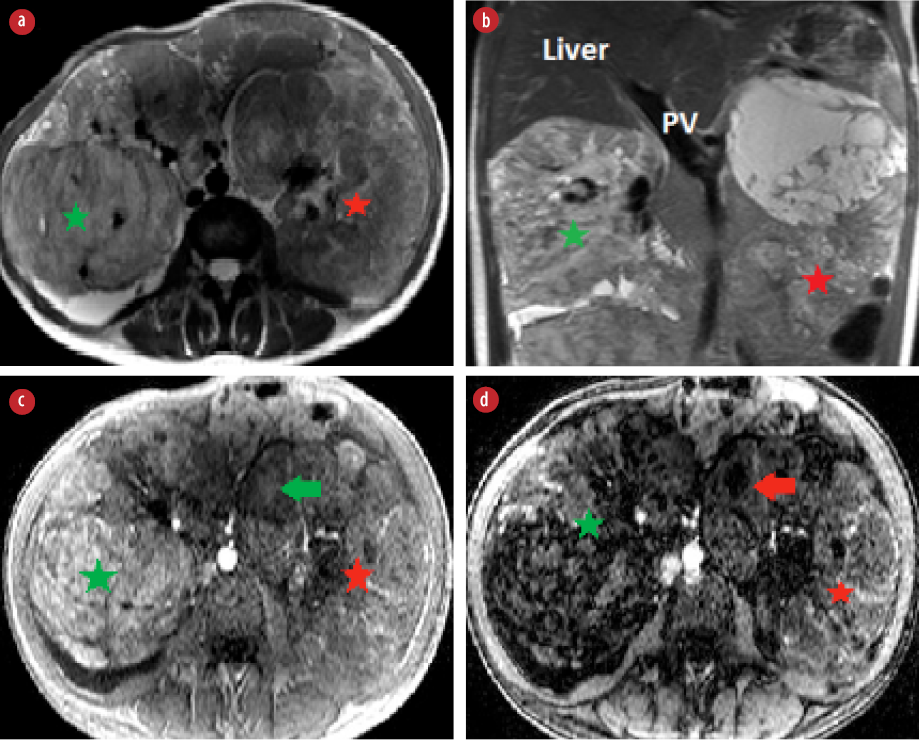
Figure 3: High-resolution chest computed tomography scan axial views. (a and b) Bilateral pulmonary nodules (red arrows). (c and d) Bilateral pulmonary cysts (green arrows).
Case report
A 31-year-old female was referred to our institute, the Royal Hospital, Muscat, Oman with a history of bilateral huge renal masses and end-stage kidney disease for further work-up and possible nephrectomy. Imaging of the renal masses at her local hospital were suggestive of bilateral AML. However, the images were not available for further review. The patient had no clinical symptoms or signs of tuberous sclerosis complex. Laboratory tests revealed impaired renal function with an estimated glomerular filtration rate (eGFR) of 11 mL/min/1.73m2 and creatinine of 443 umol/L. Complete blood count revealed low hemoglobin of 9.1 g/dL (normal range: 11–14.5 g/dL).
MRI of the abdomen showed bilateral large and heterogeneous renal masses that were replacing renal parenchyma. The right renal mass showed high signal intensity in T1- and T2-weighted images with large areas of signal drop in the out-phase sequence indicating fatty component [Figure 1]. The findings were consistent with classical renal AML. The left renal mass was heterogeneous with minimal fat component [Figure 1]. The tumor had large areas of necrosis and cystic changes. The solid component was hyperintense in both T1- and T2-weighted images. Findings of left renal mass were not typical for classical AML. Intravenous contrast was not administered because the patient had low eGFR. Dimercaptosuccinic acid scan was done to assess renal function and showed poor function of both kidneys.
The patient underwent left nephrectomy, and histology examination showed a tumor that consisted of epithelioid cells with moderate nuclear atypia along with abnormal thickened blood vessels, smooth muscle, and mature adipose tissue
[Figure 2]. More than 70% of the epithelioid cells showed atypia. The tumor had scanty mitotic figures
(1/10 hpf). Large areas of hemorrhage, necrosis, and cystic changes were detected within the neoplasm. The tumor was infiltrating the renal capsule and extending into the perinephric fat.
Chest CT was performed and revealed bilateral pulmonary nodules that are suggestive of pulmonary metastasis [Figure 3 a and b]. In addition, there were bilateral pulmonary cysts [Figure 3 c and d] with characteristic features of pulmonary LAM with more than 10 pulmonary cysts randomly distributed throughout both lungs in the absence of any other intestinal lung disease features. A follow-up positron emission tomography-CT (PET-CT) scan after six months showed stable multiple non-[18F]-fluorodeoxyglucose avid pulmonary nodules and no evidence of local recurrence. The patient was offered right nephrectomy. However, she was reluctant to undergo a second surgery.
Discussion
AML is one of the most common renal tumors classified under the group of PEComas.1 Approximately 80% of AML are sporadic, while others are seen as part of the tuberous sclerosis complex (TSC). The incidence of AML in the general population without tuberous sclerosis is 0.13%.6 LAM is a rare benign lung disorder included under PEComas tumors.1 LAM can be sporadic or more commonly associated with TSC. Sporadic LAM occurs almost exclusively in young females in their childbearing age and affects approximately 1 in 400 000 adult females. In patients with TSC, LAM affects 30–40% of adult females and is rarely encountered in adult males and children.7 The characteristic high-resolution CT (HRCT) findings of LAM are multiple (> 10), well-defined lungs cysts with preserved increased lung volume, and no other pulmonary findings of interstitial lung disease.7 Our patient met the diagnostic criteria for definite LAM with characteristic HRCT findings and renal AML. In the literature, there are few reported cases of EAML with LAM. We presented a case of EAML with definite LAM in the absence of clinical findings of TSC. The classical AML consists of a variable proportion of dysmorphic blood vessels, smooth muscles, and adipose tissue. Although benign, classical AML can have an acute presentation due to spontaneous hemorrhage and, in some cases, can be locally invasive with infiltration of adjacent veins and lymph nodes.3 The diagnosis of AML can be made on imaging by the demonstration of macroscopic fat and lack of calcification.3,5
EAML is uncommon variant of AML described by Pea et al in 1998.2,8 EAML is a highly cellular tumor that predominantly consists of sheets of atypical epithelioid cells with absent or low adipose tissue component mimicking renal cell carcinoma histologically and radiologically.3–5,9 However, the proportion of epithelioid cell component that warrants the classification as EAML is unclear, ranging from 5% to 100%.3 The 2004 World Health Organization classification of tumors classified EAML as a tumor with a malignant potential that can have an aggressive behavior such as local invasion, distant metastasis, and local recurrence after excision.10 However, even with the presence of cytological atypia, the differentiation between EAMLs with the aggressive and benign course cannot be anticipated.3,4 In the literature, four atypical histological features can be used to predict the aggressive behavior of EAML, including (a) > 70% atypical epithelioid cells; (b) > 2 mitotic figures/10 hpf; (c) atypical mitoses; and (d) necrosis. The presence of at least three of these features is considered highly suggestive of aggressive EAML.3,4 In our case, the tumor had necrosis, and more than 70% of the epithelioid cells showed atypia. However, the tumor had scanty mitotic figures (1/10 hpf) and no atypical mitosis.
There is a wide-spectrum of radiological features of EAML, reflecting its histological diversity. Imaging features that are suggestive of EAML include large size lesion that is hyperdense compared the renal parenchyma on unenhanced CT and low signal intensity in T2 weighted images reflecting the hypercellularity of the lesion, lack of fatty component, internal hemorrhage, and heterogenous enhancement on post-contrast examination with early wash-in and late washout.3–5,9,11 However, most authors agreed that the imaging findings of EAML are not specific and can overlap with the features of renal cell carcinoma and AML with minimal fat.3–5,11 Tsai et al,5 reported the absence of fat and calcification on CT in a series of five cases. In the same series, all tumors showed early enhancement. The same authors reviewed 22 cases in the literature and found that two cases had fat while the others had no fat component. Froemming et al,3 reported a series of nine cases with variable imaging findings ranging from small, well-defined lesions to large and heterogeneous lesions. Five of nine cases had small fatty component, and one had calcification. The authors of the same series concluded that the imaging features of EAML are not specific and can be confused with renal cell carcinoma and angiomyolipoma with minimal fat. However, they concluded that EAML should be considered in the differential diagnosis of a fat-poor renal lesion with no calcification, and if the renal lesion has internal hemorrhage.4 Bharwani et al,9 reported two cases of EAML that were hyperdense on CT scan with no fatty component on both CT and MRI. In our case, the MRI findings were similar to the features reported in the literature with a very large and heterogeneous left renal mass that had hyperintense signal in T2 with internal hemorrhage and minimal fat. Previous reports have shown metastasis of EAML to the liver, lung, lymph nodes, and mesentery. Our patient had a chest CT that revealed bilateral randomly distributed pulmonary nodules suggestive of pulmonary metastasis.
The management of EAML is controversial because of uncertainty about the natural history of the condition. However, because EAML has the potential for malignancy, it is often managed as renal cell carcinoma. Nephrectomy is the primary treatment of choice.12 Although some patients responded well to a single-agent doxorubicin,13 the outcomes have not been validated by long-term and large clinical trials. Adjuvant chemotherapy is not often indicated for this tumor.
Conclusion
EAML is a rare renal neoplasm with variable imaging findings that can overlap with other more common renal neoplasms. Therefore, EAML should be considered as part of the differential diagnosis of any large renal mass with the following histological features: minimal fat, necrosis, and hemorrhage.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Malinowska I, Kwiatkowski DJ, Weiss S, Martignoni G, Netto G, Argani P. Perivascular epithelioid cell tumors (PEComas) harboring TFE3 gene rearrangements lack the TSC2 alterations characteristic of conventional PEComas: further evidence for a biological distinction. Am J Surg Pathol 2012 May;36(5):783-784.
- 2. Pea M, Bonetti F, Martignoni G, et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 1998; 22:180–187.
- Froemming AT, Boland J, Cheville J, Takahashi N, Kawashima A. Renal epithelioid angiomyolipoma: imaging characteristics in nine cases with radiologic-pathologic correlation and review of the literature. AJR Am J Roentgenol 2013 Feb;200(2):W178-W186.
- 3. Tsili AC, Ntorkou A, Argyropoulou MI. Renal epithelioid angiomyolipoma associated with pulmonary lymphangioleiomyomatosis: imaging findings. J Clin Imaging Sci 2017 May;7:18.
- 4. Tsai CC, Wu WJ, Li CC, Wang CJ, Wu CH, Wu CC. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: a clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci 2009 Mar;25(3):133-140.
- 5. Fujii Y, Komai Y, Saito K, Iimura Y, Yonese J, Kawakami S, etal. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology 2008 Sep;72(3):598-602.
- Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al; Review Panel of the ERS LAM Task Force. European respiratory society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010 Jan;35(1):14-26.
- Naimi A, Riahinejad M, Mohammadizadeh F, Hosseinpour M. Pure epithelioid angiomyolipoma of kidney in tuberous sclerosis patient: a case report and review of literature. Archives of Medicine and Health Sciences 2016;4(2):222-224.
- 7. Bharwani N, Christmas TJ, Jameson C, Moat N, Sohaib SA. Epithelioid angiomyolipoma: imaging appearances. Br J Radiol 2009 Dec;82(984):e249-e252.
- 8. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart 2004;10:179-84.
- Zhu J, Li H, Ding L, Cheng H. Imaging appearance of renal epithelioid angiomyolipoma: A case report and literature review. Medicine. 2018;97(1):e9563.
- 9. Serrano Frago P, Del Agua Arias Camisón C, Gil Sanz MJ, Allué López M, Gonzalvo Ibarra A, Plaza Mas L, et al. Controversies related to epithelioid variant of renal angiomyolipoma: a review of the literature. Urology 2006 Apr;67(4):846.e3-846.e5.
- 10. Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. Malignant epithelioid angiomyolipoma (‘sarcoma ex angiomyolipoma’) of the kidney: a case report and review of the literature. Am J Surg Pathol 2001 Jan;25(1):121-126.