Common variable immunodeficiency (CVID) is a heterogeneous category and the most common clinically significant primary immunodeficiency (PID) disorder characterized by impaired B cell differentiation to memory B cell and plasma cell.1 Diagnosis of CVID is done by a marked reduction of at least two immunoglobulin (Ig) isotypes: IgG with IgA and/or IgM, and impaired specific antibody production against protein or polysaccharide antigens and vaccines.2,3 Likewise, T-cell dysfunctions are reported in approximately one-third of CVID patients and contribute to the more variable clinical manifestations of the disease such as the development of opportunistic or unusual infections.2,4–6 Several reports proposed that CVID represents a heterogeneous disease spectrum with a variety of clinical presentations including autoimmune disorders (AID), lymphoproliferative disease, enteropathy, malignancy, and recurrent bacterial and viral infections.7,8
Recurrent infections are among the first and the most common clinical manifestation of the disease. Acute and chronic infections are a leading cause of morbidity in patients with CVID.7 Approximately, all CVID patients presented with recurrent upper and/or lower respiratory tract infections, including otitis media, sinusitis, bronchitis, and pneumonia.6,9,10 Recurrent infections especially respiratory tract infections (20–96%) and gastrointestinal infections (30–88%) are associated with a low subset of B cells, specifically reduced isotype-switched memory B cells and reduced immunoglobin levels.5,11–14
In this review, we sought to evaluate the existing evidence for rates of infectious complications, and performed a cumulative analysis of all studies reporting these complications. To the best of our knowledge, this is the first systematic review examining the infectious findings in CVID.
Methods
This systematic review and meta-analysis is carried out based on PRISMA statement guidelines.15
Our search strategy composed of three components: (1) comprehensive searching of international and national electronic databases for published documents, (2) hand-searching of the reference section of the retrieved scientific documents, and (3) contacting experts in the field in order to assess unavailable papers.
We performed a comprehensive search using the Scopus, PubMed, and Web of Science databases to gather English articles published up to January 2018. Search strategy keywords and MeSH terms were categorized in two groups and combined: (1) ‘CVID’, ‘common variable immunodeficiency’, ‘hypogammaglobulinemia’, ‘primary antibody deficiency’; and (2) ‘infection’, ‘pneumonia’, ‘sinusitis’, ‘otitis’, ‘meningitis’, ‘diarrhea’, ‘hepatitis C’, ‘skin infection’, ‘gastrointestinal infection’, ‘candidiasis’, ‘upper respiratory tract infection’, ‘tonsillitis’, ‘pharyngitis’, ‘abscess’, ‘conjunctivitis’, ‘CNS infection’, ‘sepsis’, ‘septic arthritis’, ‘osteomyelitis’, ‘bacterial infection’, ‘viral infection’ ‘parasitic infection’, or ‘fungal infection’.
Screening of the gathered documents was done in two steps. We first screened by title and abstract to exclude all irrelevant studies, and then assessed the full texts for eligibility criteria. The inclusion criteria were: (1) English-language studies; (2) study design as prospective or retrospective cohort, cross-sectional, case series, or case-control studies; (3) studies conducted on human subjects; (4) the targeted population were those who met the international (PAGID and/or European Society for Immunodeficiency (ESID) diagnostic criteria for diagnoses of CVID; (5) their subject of the evaluation was the epidemiological, clinical, and immunological features of patients; and (6) their primary or alternative outcome of interest was infection incidence or prevalence.
Additionally, to gain further insight into the characteristics of CVID patients who developed infections, data from all studies which describe in detail the characterization of CVID patients either with infections or without infections was obtained. Review articles, studies using animal models, and studies regarding other types of PID than CVID were all excluded. For studies with overlapping data, only the study with the largest patient cohort was included. Both the steps were done independently by two reviewers, and discrepancies between the reviewers were resolved by the third reviewer.
Two authors extracted data independently from the included studies based on title and abstracts in a standardized Microsoft Excel spreadsheet. Any disagreements were resolved by discussing and consensus with a third author. The following data were collected from all identified studies: name of the first author, published year, the country of origin of the study, study design, the population characteristics, demographics, clinical, and immunologic data. The medical records of all papers were gathered if a case was reported in more than one study.
Aggregated data analysis was done with simple pooled data to provide an overall summary of subgroup data or data from a number of related studies. Data were combined without being weighted, and the analysis was performed as if the data were derived from a single sample. Central and descriptive statistics were reported for quantitative data. For variables with skewed distribution, median and interquartile range (IQR) were reported as the index of data dispersion. Analytical analyses was performed using Mann-Whitney U, chi-square, and Fisher’s exact tests.
Meta-analysis was performed for the prevalence data on infections and various types of infections in the studies of CVID. Given the expected heterogeneity between studies, a meta-analysis was performed using a random-effects model to account for inter-study variation. Heterogeneity was assessed using the I-square (I2) statistic, which describes the percentage of variation between studies that is due to heterogeneity rather than chance. Data analysis was conducted STA
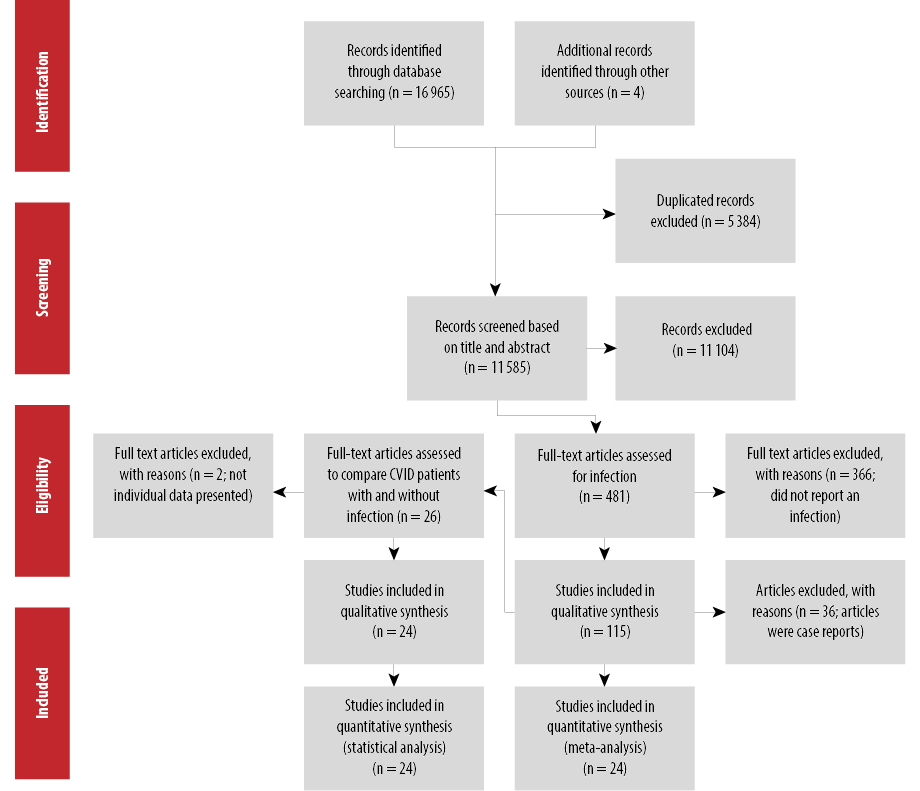
Figure 1: Flow diagram of the systematic review and meta-analysis for infections in CVID.
TA v.14 software (Stata-Crop, College Station, TX).
Results
The results of the literature search and selection process are shown in Figure 1. A total of 16 969 articles were retrieved from the initial search from which 5384 were duplicated studies. After screening 11 585 studies for titles and abstracts, 481 articles were selected, and the full texts were assessed. Of the 481 studies, 366 studies did not meet the eligibility criteria, from which most did not present the data of infections independently, and 36 case reports were excluded. Finally, 79 studies met the inclusion criteria and were analyzed.4–14,16–83 The characteristics of the included studies in this systematic review are depicted in Table 1. The sample sizes of CVID patients varied from 4 to 2212 in a study from the ESID. The studies were conducted in 19 countries with most originating from the US (n = 11), Iran (n = 12), and France (n = 9). The oldest study was carried out in 1972 and the latest in 2018.
Table 1: Main characteristics of the included studies on the prevalence of infection in common variable immunodeficiency patients.
|
Desjardins, M. (2018) |
Canada |
Case-control |
McGill University Health Centre and Centre Hospitalier Universitaire de Québec |
42 (M = 14,
F = 28) |
Mean age of onset = 18.1
Mean age of diagnosis = 32.1 |
26 (61.9)
(45.6–76.4) |
|
|
|
URTI: 33 (78.6)
(64.1–88.3)
Sepsis: 2 (4.8)
(0.6–16.2)
CNS infection: 2 (4.8)
(0.6–16.2) |
|
Erazo-Borrás, L. (2017) |
Colombia |
Case-control |
- |
22 (M = 12,
F = 10) |
Age of onset median = 5 (0.5-42)
Median age at diagnosis = 26 (6–49)
Median current age
= 37 (13–63) |
16 (72.7) (49.8–89.3) |
10 (45.5)
(24.4–67.8) |
11 (50.0)(28.2–71.8) |
7 (31.8)(13.9–54.9) |
SI: 8 (36.4)
(17.2–59.3)
Pharyngitis: 7 (31.8)
(13.9–54.9)
Tonsillitis: 7 (31.8)
(13.9–54.9) |
|
Azizi, G. (2018) |
Iran |
Case-control |
National registry of PID
Children’s Medical Center affiliated to Tehran University of Medical Sciences |
72 (M = 41,
F = 31) |
Median current age = 24 (33.5–16.25)
Median age of onset = 4.0 (11.0–1.0)
Median age of diagnosis = 13.50 (28.75–7.0) |
52 (72.2)
(60.4–82.1) |
35 (48.6)
(36.7–60.7) |
44 (61.1)
(48.9–72.4) |
|
Conjunctivitis: 10 (13.9)
(6.9–24.1)
CNS infection: 8 (11.1)
(4.9–20.7) |
|
Azzu, V. (2017) |
UK |
Case series |
|
4 (M = 3,
F = 1) |
Average age at diagnosis = 26 |
1 (25.0)
(4.6–69.9) |
|
|
|
URTI: 1 (25.0)
(4.6–69.9)
Abscess: 2 (50.0)
(15.0–85.0) |
|
Sanchez, L. (2017) |
US |
Cohort |
United States Immunodeficiency Network (USIDNET) database |
349 (M = 190,
F = 159) |
Age range: 3–91
Early onset (2–10) = 110 patients
Adolescent onset (11–17) = 83 patients
Adult onset (> 18) = 264 patients
Age of diagnosis range: 2–76.9 |
299 (65.4)
(60.9–69.8) |
205 (44.9)
(40.2–49.5) |
357 (78.1)
(74.0–81.8) |
86 (18.8) (15.3–22.7) |
Candida: 68 (14.9)
(11.7–18.5)
SI: 86 (18.8)
(15.3–22.7)
Sepsis: 38 (8.3)
(6.0–11.2)
Abscess: 36 (7.9)
(5.6–10.7)
Osteomyelitis: 4 (0.9)
(0.2–2.2)
Conjunctivitis: 54 (11.8)
(9.0–15.1)
Pharyngitis: 73 (16.0)
(12.7–19.7)
Tonsillitis: 23(5.0)
(3.2–7.5)
CNS infection: 29 (6.3)
(4.3–9.0) |
|
Valizadeh, A. (2017) |
Iran |
Cohort |
Children’s Medical Center Hospital, Pediatrics Center of Excellence |
120 (M = 67,
F = 53) |
Median age, year (IQR) =
20 (14–28)
Age of onset =
2 (0.6–6)
Age of diagnosis median (IQR) =
9 (4–15.7) |
42 (35.0) (26.5–44.2) |
|
|
|
SI:24 (20.0)
(13.3–28.3)
URTI: 39 (32.5)
(24.8–41.3)
Abscess: 3 (2.5)
(0.5–7.1)
Conjunctivitis: 36 (30.0)
(22.0–39.0)
Septic arthritis: 10 (8.3)
(4.1–14.8)
CNS infection: 4 (3.3 )
(0.9–8.3) |
|
Friedmann, D. (2017) |
Germany |
Case-control |
- |
58 (M = 24,
F = 34) |
Age range: 19–75 |
|
|
|
25 (43.1)
(30.2–56.8) |
|
|
Selenius, J. (2017) |
Finland |
Cross-sectional |
Hospital District of Helsinki and Uusimaa
Adult Immunodeficiency Unit of Helsinki University Hospital
clinics in Kymenlaakso Social and Health Services (Carea) and South Karelia Social and Health Care District (Eksote) |
132 (M = 67,
F = 65) |
Age range at diagnosis: 9–74
Age range at the time of study: 20–84 |
13 (50.0)
(29.9–70.1) |
|
|
|
|
|
Çalişkaner, A. (2016) |
Turkey |
Cohort |
Adult immunology clinic in the Central Anatolia region of Turkey, |
25 (M = 12,
F = 13) |
Mean age =
36.6 ± 13.4
Delay in diagnosis was 107 ± 95.6 months |
19 (76.0)
(54.9–90.6) |
|
3 (12.0)
(4.2–30.0) |
2 (8.0)
(1.0–26.0) |
URTI: 3 (12.0)
(4.2–30.0) |
|
Furudoï, A. (2016) |
France |
Cohort |
10 patients were registered
in the French DEFI cohort and seven patients were followed up in the Department of Internal Medicine, Haut-Le vveˆque Hospital |
17 (M = 8,
F = 9) |
Age of onset mean = 20.1
Age of diagnosis mean = 34.9 |
11 (68.8)
(41.3–89.0) |
7 (43.8) (19.8–70.1) |
1 (6.3)
(0.2–30.2) |
|
CNS infection: 3 (18.8)
(4.0–45.6) |
|
Janssen, W.
(2017) |
Netherlands |
Cohort |
Department of Pediatric Immunology and Infectious Diseases, Laboratory of Translational Immunology, Wilhelmina Children’s Hospital |
55 (M = 30,
F = 25) |
- |
29 (43.6) (31.4–56.7) |
|
|
|
URTI: 29 (43.6)
(31.4–56.7) |
|
Kutukculer, N.
(2016) |
Turkey |
Case-control |
Ege University Pediatric Immunology Department |
20 (M = 17, F = 3) in case (CVID) |
Mean age of onset = 7
Mean age of diagnosis = 8 |
|
|
|
|
|
|
Mokhtari, M.
(2016) |
Iran |
Retrospective Cohort |
Children’s Medical Center (Pediatrics Center of Excellence) |
185 total 113 studied (M = 69, F = 44) |
Mean age of onset = 5.9 ±4.7
Mean age of diagnosis = 12.7±11.3 |
93 (82.3)
(74.0–88.8) |
|
78 (69.0)
(59.6–77.4) |
|
CNS infection: 12 (10.6)
(5.6–17.8) |
|
Yazdani, R.
(2016) |
Iran |
Case-control |
Children’s Medical Center (Pediatrics Center of Excellence) |
30 (M = 20, F = 10) |
Mean age =
23.43 ± 11.58
Mean age of onset = 6.32 ± 8.57
Mean diagnostic delay = 6.21 ± 5.43 |
23 (76.7)
(57.7–90.1) |
16 (53.3)
(34.3–71.7) |
19 (63.3)
(43.9–80.1) |
|
|
|
Lin, L.
(2015) |
China |
|
Five cases from Peking University First Hospital
35 cases from China National Knowledge Infrastructure and Wan Fang Database |
40 (M = 30,
F = 10) |
Median age at onset = 11 (range: 4–51)
Median age at diagnosis = 14.5 (range: 5–66). Average time of delay in diagnosis = 5.3 years
(range: 1–41) |
28 (70.0)
(53.5–83.4) |
7 (17.5)
(7.3–32.8) |
5 (12.5)
(4.2–26.8) |
12 (30.0)
(16.6–46.5) |
SI:
1 (2.5)
(0.1–13.2)
Abscess:
2 (5.0)
(0.6–16.9)
CNS infection:
1 (2.5)
(0.1–13.2) |
|
Lougaris, V.
(2016) |
Italy |
|
Pediatrics Clinic, University of Brescia, Italy |
15(M = 9,
F = 6) |
Mean age at diagnosis = 15 Mean age at the time the study = 27.3 |
8 (53.3)
(26.6–78.7) |
|
|
|
|
|
Musabak, U.
(2017) |
Turkey |
|
- |
31 (M = 19,
F = 12) |
Current age median = 28
Age at diagnosis median =23
Delay in diagnosis median = 14 |
20 (64.5)
(45.4–80.8) |
14 (45.2)
(27.3–64.0) |
26 (83.9)
(66.3–94.5) |
12 (38.7) (21.8–57.8) |
Candida:
3 (9.7)
(2.0–25.8)
SI:
2 (6.5)
(0.8–21.4)
CNS infection:
2 (6.5)
(0.8–21.4) |
|
Arshi, S.
(2016) |
Iran |
Retrospective Cohort |
The Allergy and Clinical Immunology department of Rasol E Akram Hospital of Iran University of Medical sciences, Tehran, Iran |
47 (M = 47,
F = 0) |
Mean age = 27 (range: 4–63)
Mean follow-up time = 6.8
(range: 0.5–23).
Mean age of onset = 11.2
(range: 1–32)
Mean diagnostic delay = 9 |
|
|
|
12 (25.5) (13.9–40.3) |
SI:
16 (34.0)
(20.9–49.3)
Abscess:
12 (25.5)
(13.9–40.3)
Osteomyelitis:
10 (21.3)
(10.7–35.7)
CNS infection:
6 (12.8)
(4.8–25.7) |
|
Dong, J.
(2016) |
China |
Retrospective cohort |
Inpatients or outpatients of the Affiliated Hospital(the largest tertiary referral center) of Qingdao University, |
8
(M = 5, F = 3) |
Mean age at onset = 32.5 ± 12.6
(range: 15–49)
Mean age at diagnosis =
43 ± 13.7 year
Mean diagnostic delay = 10.5 |
5 (62.5)
(24–591.5) |
1 (12.5)
(0.3–52.7) |
|
|
|
|
Gathmann B.
(2014) |
European Society |
Cohort |
28 medical centers contributing to the European Society for Immunodeficiencies |
2212
(M = 1081,
F = 1131) |
- |
288 (31.9)
(28.9–35.1) |
|
|
|
CNS infection:
36 (4.0 )
(2.8–5.5) |
|
Berrón-Ruiz, L.
(2014) |
Mexico |
Cohort |
Instituto Nacional de Pediatría and Centro Médico Nacional ‘‘La Raza’’ Instituto Mexicano del Seguro Social, Mexico City |
16 (M = 6,
F = 10) |
Mean age at onset = 12.2
Mean age at diagnosis = 14.6 |
14 (87.5)
(61.7–98.4) |
7 (46.7)
(21.3–73.4) |
12 (75.0)
(47.6–92.7) |
12 (75.0)
(47.6–92.7) |
CNS infection:
16 (100)
(79.4–100) |
|
Maglione, P.
(2014) |
US |
Retrospective analysis |
electronic medical records from Mount Sinai Hospital (New York, New York) |
61 (M = 27,
F = 34) |
Median age of 47 years (range: 14–89) |
33 (54.1)
(40.8–66.9) |
|
|
|
|
|
Ramírez-Vargas, N.
(2014) |
Mexico |
Cohort |
Immunol ogy Division of seven different reference centres in Mexico |
43 (M = 23,
F = 20) |
Age of onset median = 13.7
Age of diagnosis median = 19 |
36 (83.7)
(69.3–93.2) |
21 (48.8)
(33.3, 64.5) |
36 (83.7)
(69.3–93.2) |
30 (69.8)
(53.9–82.8) |
Candida:
2 (4.7)
(0.6–15.8)
Sepsis:
2 (4.7)
(0.6–15.8)
Abscess:
2 (4.7)
(0.6–15.8)
Osteomyelitis:
2 (4.7)
(0.6–15.8)
CNS infection:
7 (16.3)
(6.8–30.7) |
|
Agondi, R.
(2013) |
Brazil |
Cross-sectional |
Primary Immunodeficiency Outpatient Clinic of the Division of Clin- ical Immunology and Allergy, University of Sa ̃o Paulo, from January 2009 to December 2011 |
72 (M=35, F=37) |
Mean age at onset = 13.8 ±10.7
Mean age at diagnosis =
28.6 ± 12.5
Mean time to diagnosis =
14.8 ± 12.0 years |
53 (73.6)
(61.9–83.3) |
|
45 (62.5)
(50.3–73.6) |
|
CNS infection:
5 (6.9)
(2.3–15.5) |
|
Mohammadinejad P.
(2012) |
Iran |
Cohort |
Children medical center affiliated to the Tehran university of medical science |
69 (M=34, F=35) |
Mean time of follow-up =
5.2 ± 4.3 years
Mean diagnostic delay = 4.4 ± 3.6 years |
53 (76.8 )(65.1–86.1) |
10 (14.5)
(7.2–25.0) |
41 (59.4)
(46.9–71.1) |
40 (58.0) (45.5–69.8) |
SI:
10 (14.5)
(7.2–25.0)
URTI:
44 (63.8)
(52.0–74.1)
Sepsis:
2 (2.9)
(0.4–10.1)
Abscess:
14 (20.3)
(11.6–31.7)
Osteomyelitis:
1 (1.4)
(0.0–7.8)
Conjunctivitis:
17 (24.6)
(15.1–36.5)
Pharyngitis:
5 (7.2)
(2.4–16.1)
Septic arthritis:
10 (14.5)
(7.2–25.0)
CNS infection:
6 (8.7)
(3.3–18.0) |
|
Bayry, J.
(2011) |
France |
Case-Control |
- |
10 (M = 8,
F = 2) |
Mean age = 32.5 (23–66) |
3 (30.0)
(6.7–65.2) |
1 (10.0)
(0.3– 44.5) |
3 (30.0)
(6.7–65.2) |
1 (10.0)
(0.3–44.5) |
Candida:
3 (30.0)
(6.7– 65.2)
Conjunctivitis:
1 (10.0)
(0.3–44.5) |
|
Al-Herz, W.
(2011) |
Kuwait |
Cohort |
Allergy and Clinical Immunology Unit, Department of Pediatrics (Al-Sabah Hospital), and the Pediatric Dermatology Unit of the National Dermatology Center between January 2004 and December 2009 |
128 (5 cvid) |
Mean age at onset = 0.89 ± 1.34
Mean age at diagnosis =
3.16 ± 3.48 |
|
|
|
|
SI:
1 (20.0)
(0.57–1.6) |
|
Malamut, G.
2010 |
France |
Retrospective cohort |
Ten referral centers in France (six gastroenterology departments, two internal medicine departments, one hematology department, one clinical immunology department), between January 1962 and July 2004 |
50 (M=26, F=24) |
Mean age at onset = 34.5 ± 14.3
Mean age at diagnosis =
36.8 ± 15.6 |
|
|
|
|
|
|
Aghamohammadi, A.
(2010) |
Iran |
Retrospective |
The Immunodeficiency Clinic at the Children’s Medical Center affiliated to Tehran University of Medical Sciences |
76 (M = 43,
F = 33) |
Age at study = 17 (2–59)
Age of onset = 2 (0.5–46)
Age of diagnosis =9 (2–54)
Diagnostic delay = 5 (1–32) years |
59 (77.6)
(66.6–86.4) |
|
|
|
|
|
Ardeniz, O.
(2010) |
Turkey |
Cohort |
Ege University Medical Faculty Internal Medicine Division of Allergy and Clinical Immunology |
23(M = 13,
F = 10) |
Median age of onset = (F: 12.5 and
M: 15)
Median age of diagnosis = (F: 33 and M: 28 ) |
14 (60.9)
(38.5–80.3) |
21 (91.3)
(72.0–98.9) |
21 (91.3)
(72.0–98.9) |
|
Hep C:
1 (4.3)
(0.1–21.9)
Sepsis:
1 (4.3)
(0.1–21.9)
CNS infection:
2 (8.7)
(1.1–28.0) |
|
Carvalho, K.
(2010) |
Brazil |
Case-control |
Division of Pediatric Clinical Immunology located at the Federal University of Sao Paulo |
17 (M = 7,
F = 10) |
Median age at diagnosis (IGR) = 22 (IQR = 13.26)
Median age at first symptoms (IQR) = 12 ( 3.16) |
15 (88.2)
(63.6–98.5) |
6 (35.3)
(14.2–61.7) |
11 (64.7)
(38.3–85.8) |
|
|
|
Salehzadeh, M.
(2010) |
Iran |
Cohort |
Division of Allergy and Clinical Immunology of Children’s Medical Center Hospital |
24 (M = 17,
F = 7) |
Median age at diagnosis (IQR) = 102.5 months (2–43 years)
Median diagnostic delay (IQR) = 63.5 months (3–477 months) |
21 (87.5)
(67.6–97.3) |
16 (66.7)
(44.7–84.4) |
19 (79.2)
(57.8–92.9) |
21 (87.5)
(67.6–97.3) |
Candida:
5 (20.8)
(7.1–42.2)
SI:
8 (33.3)
(15.6–55.3)
Abscess:
5 (20.8)
(7.1–42.2)
Conjunctivitis:
8 (33.3)
(15.6–55.3)
Septic arthritis:
5 (20.8)
(7.1–42.2) |
|
Van de ven, A.
(2010) |
Netherlands |
Cohort |
Thirty-eight pediatric CVID patients of the Wilhelmina ’Children’s Hospital in Utrecht, The Netherlands, were included |
38 (M = 32,
F = 6)
9 |
Mean age at diagnosis =
5.5 ± 2.5 |
2 (22.2)
(2.8–60.0) |
|
|
2 (22.2) (2.8–60.0) |
URTI:
7 (77.8)
(45.3–93.7)
Sepsis:
2 (22.2)
(2.8–60.0)
CNS infection: 1 (11.1)
(0.3–48.2) |
|
Yong, P.
(2010) |
US |
Cohort |
Childrens Hospital of Philadelphia |
24 (M = 14,
F = 10) |
Age of onset ≥ 2
Median age of diagnosis = 84 |
11 (45.8)
(25.6–67.2) |
12 (50.0)
(29.1–70.9) |
12 (50.0)
(29.1–70.9) |
|
Sepsis:
3 (12.5)
(2.7–32.4)
CNS infection:
1 (4.2)
(0.1–21.1) |
|
Mamishi, S.
(2010) |
Iran |
Retrospective cohort |
Children’s Medical Center Hospital |
26 (M = 15,
F = 11) |
Mean age =
6.87 ± 4.15 |
16 (61.5)
(40.6–79.8) |
20 (76.9)
(56.4–91.0) |
|
20 (76.9)
(56.4–91.0) |
Hep C:
1
Sepsis:
3 (11.5)
(2.4–30.2)
Septic arthritis:
2 (7.7)
(0.9–25.1) |
|
Huck, K.
(2009) |
Germany |
Case-control |
Pediatric immunodeficiency clinic in Düsseldorf, Germany (From 1997–2007) |
16 (M = 8,
F = 8) |
Mean age at diagnosis = 9 years and 9 months,
Mean age at onset = 4 years and 8 months Diagnostic delay = 5 years |
10 (90.9)
(58.7–99.8) |
7 (43.8)
(19.8–70.1) |
6 (37.5)
(15.2–64.6) |
3 (27.3)(6.0–61.0) |
SI:
1 (9.1)
(1.6–37.7)
Osteomyelitis: 2 (18.2)
(2.3–51.8)
Conjunctivitis: 1 (9.1)
(0.2–41.3)
Tonsillitis: 2 (18.2)
(2.3–51.8)
Septic arthritis: 1 (9.1)
(0.2–41.3) |
|
Llobet, M.
(2009) |
Spain |
Retrospective cohort |
The University ChildrenÕs Hospital Vall dÕHeb- ron, Barcelona |
22 (M = 15,
F = 7) |
Median age at diagnosis = 7.8 (Range: 2.5–16 years) |
15 (68.2) (45.1–86.1) |
|
|
8 (36.4)(17.2–59.3) |
SI: 5 (22.7)
(7.8–45.4)
URTI: 11 (50.0)
(30.7–69.3)
Sepsis: 2 (9.1)
(1.1–29.2) |
|
Urschel, S.
(2009) |
Germany |
Retrospe ctive cohort |
Pediatric Immunology and Infectious Diseases, University Children’s Hospital, Ludwig Maximilians University |
32 (M = 15,
F = 17) |
Median age at diagnosis = 10.4 |
25 (78.1)
(60.0–90.7) |
22 (68.8)
(50.0–83.9) |
25 (78.1)
(60.0–90.7) |
10 (31.3)
(16.1–50.0) |
SI: 7 (15.9)
(6.6–30.1)
Sepsis: 5 (15.6) (5.3–32.8)
Conjunctivitis: 3 (9.4)
(2.0–25.0)
CNS infection: 8 (25.0)
(11.5–43.4) |
|
Yu, G.
(2009) |
USA |
Case-control |
Stanford Hospital and Clinics and Lucile Packard Children’s Hospita |
14 (M = 8,
F = 6) |
Mean age = 32 (range: 6–67) |
1 (7.1)
(0.2–33.9) |
|
|
|
|
|
Melo, K. M.
(2009) |
Brazil |
Case-control |
Recruited at the Division of Clinical Immunology at UNIFESP (Sao Paulo, Brazil) |
16 (M = 6,
F = 10) |
Median age at diagnosis = 22 (IQR = 13–26)
Median age of onset = 12 (IQR = 3–16)
Median diagnostic delay = 9
(IQR = 4–12) |
12 (75.0)
(47.6–92.7) |
|
|
|
|
|
Malphettes, M.
(2009) |
France |
Cohort |
The French DEFI study (41 Centers) |
313 (285 CVID +28 LOCID) 285CVID (M = 119, F = 166) |
Median age of onset = 19 |
|
|
|
|
Candida: 3; 1.0 (0.2, 2.8)
URTI: 258; 82.4 (77.8, 86.2) |
|
Aydogan, M.
(2008) |
Turkey |
Cohort |
Division of Pediatric Allergy and Immunology at Marmara University Medical Faculty |
10 (M = 6,
F = 4) |
Age of onset median = 4
Age of diagnosis median = 9.4 |
10 (100)
(69.2–100.0) |
7 (70.0)
(34.8–93.3) |
7 (70.0)
(34.8–93.3) |
|
|
|
Oksenhendler, E.
(2008) |
France |
Cohort |
Department of Clinical Immunology, Hospital Saint-Louis in Paris |
252 CVID= (M = 110,
F = 142) + 89 other |
Median age of onset = 19
Median age of diagnosis = 33.9 |
147 (58.3)
(52.0–64.5) |
|
160 (63.5)
(57.2–69.4) |
67 (26.6) (21.2–32.5) |
Candida: 2; 0.8 (0.1, 2.8)
Hep C: 3; 1.2 (0.2, 3.4)
URTI: 175; 69.4 (63.5, 74.8)
Sepsis: 33; 13.1 (9.2, 17.9)
CNS infection: 20; 7.9 (4.9, 12.0) |
|
Ramyar, A.
(2008) |
Iran |
Retrospective analysis |
Children Medical Center Hospital as the referral center for primary immunodeficiency disorders |
7 (M = 5,
F = 2) |
- |
5 (71.4)
(29.0–96.3) |
5 (71.4)
(29.0, 96.3) |
4 (57.1)
(18.4–90.1) |
|
Abscess: 7 (100)
(64.6–100) |
|
Rezaei, N.
2008 |
Iran |
|
Iranian Primary Immunodeficiency Registry- recruited from among the medical personnel of the Children’s Medical Center Hospital |
25 (M = 18,
F = 7) |
Median age at onset = 13 months (range : 1–480)
Median age at diagnosis = 97 months (range: 18–513)
Median diagnostic delay = 45 months (2-452) |
24 (96.0)
(79.6–99.9) |
17 (68.0)
(46.5–85.1) |
19 (76.0)
(54.9–90.6) |
13 (52.0) (31.3–72.2) |
Candida: 6 (24.0)
(9.4–45.1)
Osteomyelitis: 1 (4.0)
(0.1–20.4)
Conjunctivitis: 9 (36.0)
(18.0–57.5)
Septic arthritis: 6( 24.0)
(9.4–45.1)
CNS infection: 1 (4.0)
(0.1–20.4) |
|
Sève, P.
(2008) |
France |
Retrospective cohort |
Department of Internal Medicine, Hotel Dieu, 1 place de l’Hospital |
18 (M = 9,
F = 9)
14 |
Median age of onset = 27.5 years Median age of diagnosis = 6 |
|
|
|
|
URTI: 8 (57.1)
(32.6–78.6) |
|
Ward, C.
(2008) |
UK |
Cohort |
Department
of Immunology at the Oxford Radcliffe Hospitals |
108 |
- |
|
|
|
|
Hep C: 6 (12.8)
(4.8–25.7) |
|
Johnston, D. T.
(2007) |
US |
Retrospective analysis |
Consecutive patients with CVID9 who had attended the Adult Primary Immunodeficiency Clinic, University of Alabama at Birmingham |
55 (M = 28,
F = 27) |
- |
34 (61.8)
(47.7–74.6) |
|
41; 74.5
(61.0, 85.3) |
|
|
|
Quinti, I.
(2007) |
ITALY |
Cohort |
26 Italian Centers belonging to the Italian Primary Immunodeficiency Network |
224 (M = 111, F = 1 13) |
Mean age of onset = 26.6
Mean age of diagnosis = 8.9 |
110 (49.1)
(42.4–55.9) |
87 (38.8)
(32.4–45.6) |
121 (54.0)
(47.3–60.7) |
|
Candida:20 (8.9)
(5.5–13.5)
Hep C: 15 (34.9)
(21.0–50.9)
Sepsis: 5 (2.2)
(0.7–5.1)
Septic arthritis: 5 (2.2)
(0.7–5.1)
CNS infection: 3 (1.3)
(0.3,–3.9) |
|
Khodadad, A.
2007 |
Iran |
Retrospective cohort |
Iranian Primary Immunodeficiency Registry |
39 (M = 24,
F = 15) |
Mean age = 16 ± 12 |
|
|
|
|
|
|
Alachkar, H.
(2006) |
UK |
Cohort |
regional primary immunodeficiency clinics in Manchester, United Kingdom, in 2004—5 |
34 |
- |
33 (97.1) (84.7–99.9) |
|
|
|
|
|
Ogershok, P..
(2006) |
West Virginia |
Cohort |
children younger than 18 years who presented with CVID between the years of 1992 to 2005 to the West Virginia University immunology clinic after approval of the local institutional review board |
12 (M = 8,
F = 4) |
Mean age of onset = 8
Mean age of diagnosis = 8.33 |
7 (58.3)
(27.7–84.8) |
8 (66.7)
(34.9–90.1) |
9 (75.0)
(42.8–94.5) |
3 (25.0) (5.5–57.2) |
|
|
Carbone, J.
2006 |
Spain |
Case-control |
- |
14 (M = 8,
F = 6) |
Mean age = 37.4 (21–68) |
11 (78.6)
(49.2–95.3) |
2 (14.3) (1.8–42.8) |
5 (35.7) (12.8–64.9) |
7 (50.0) (23.0–77.0) |
Conjunctivitis: 2 (14.3)
(1.8–42.8) |
|
Viallard, J.
2006 |
France |
Case-control |
- |
50 (M = 19,
F = 31) |
Median age = 38 (17–77) |
23 (46.0
(31.8–60.7) |
5 (10.0) (3.3–21.8) |
40 (80.0)
(66.3–90.0) |
9 (18.0) (8.6–31.4) |
Pharyngitis: 16 (32.0)
(19.5–46.7) |
|
Fevang, B.
(2005) |
Norway |
Case-control |
Section of Clinical Immunology and Infectious Diseases, Medical Department, Rikshospitalet University Hospital, Oslo |
71 (M = 40,
F = 31) |
Median age = 44 (29–56)
Median age of onset = 18 (6–35)
Median age at diagnosis = 36 (20–49) |
38 (53.5) (41.3–65.5) |
|
|
|
URTI: 46 (64.8)
(53.2–74.9) |
|
Thickett, K.
(2002) |
Birmingham |
Retrospective cohort |
During 1997/1998, patients with CVID attending the regional immunology clinic |
47 (M = 27,
F = 20) |
Median age (range) = 45.5 (22–81)
Median age at diagnosis (range) = 35.0 (5–72)
Median time from first symptoms to diagnosis (range) = 4.0 (0.8–45) years |
9 (19.1)
(9.1–33.3) |
|
22 (46.8) (32.1–61.9) |
|
|
|
Cunningham-Rundles, C.
2002 |
US |
Cohort |
- |
5 (M = 1,
F = 4)
or
248 |
Mean age at diagnosis = 33 (range: 13–46) |
2 (40.0)
(5.3–85.3) |
1 (20.0)
(0.5–71.6) |
|
|
URTI: 1 (20.0)
(3.6–62.4)
Sepsis: 3 (1.2)
(0.3–3.5)
Abscess: 4 (1.6)
(0.4–4.1)
Osteomyelitis:
2 (0.8)
(0.1–2.9)
Septic arthritis: 2 (0.8)
(0.1–2.9) |
|
Guazzi, V.
(2002) |
Italy |
Case-control |
Seventeen patients affected by CVID and followed at the Division of Allergy and Clinical Immunology, University of Rome ‘La Sapienza’, were included |
17 (M=8, F=9) |
Age range = 24–61 (mean = 47) |
|
|
|
|
Hep C:
3 (17.6)
(3.8–43.4) |
|
Quinti, I.
(2002) |
16 countries |
Cross-sectional |
A questionnaire was sent to 125 clinical centers from 26 European countries |
952 |
Mean age (range) = 47.8 (10–81) |
|
|
|
|
Hep C:
50 (5.3)
(3.9–6.9) |
|
Kainulainen, L.
(2001) |
Finland |
Cohort |
The Finnish Social Insurance Institute maintains a central register of patients with primary hypogammaglobulinemia |
97 (M = 54, F = 43)
or
18 |
Median age = 33 years
Median age at diagnosis = 32 (0.5–73.0)
Duration of symptoms before diagnosis (median) = 5 years (0.2–37.0) |
63 (66.3)
(55.9–75.7) |
28 (29.5)
(20.6, 39.7) |
57 (60.0)
(49.4, 69.9) |
|
Conjunctivitis:
9 (9.5)
(4.4–17.2)
CNS infection:
6 (6.3)
(2.4–13.2) |
|
Martinez Garcia, M. A.
(2001) |
Spain |
Cross-sectional |
Departments of Allergology, Internal Medicine, Pediatrics and Pneumology of hospital Universitario La Fe, Valencia, Spain |
19 (M = 12, F = 7) |
Mean age at onset = 14.7
Mean age at diagnosis = 23.2 |
14 (73.7)
(48.8–90.9) |
12 (63.2)
(38.4–83.7) |
12 (63.2)
(38.4–83.7) |
|
Candida:
8 (42.1)
(20.3–66.5)
URTI:
19 (100)
(83.2–100.0) |
|
Nijenhuis, T.
(2001) |
Netherlands |
Case series |
Starting with three affected family members (II:17, III:14 and IV:6; Fig. 1), we set out to analyze the pedigree |
6 (M = 2, F = 4) |
- |
3 (50.0)
(18.8–81.2) |
|
|
1 (16.7)
(0.4–64.1) |
SI:
1 (16.7)
(0.4–64.1)
URTI:
3 (50.0)
(18.8–81.2) |
|
Bjóro, K.
(1999) |
Norway |
Case-control |
Section of Clinical Immunology and Infectious Disease, Department of Medicine, The National Hospital, Oslo |
58 |
Median age = 44 (14–76) |
|
|
|
|
Hep C:
6 (10.3)
(3.9–21.2) |
|
Aukrust, P.
(1999) |
Norway |
Case-control |
- |
20 (M = 8, F = 12) |
Median age =43 (25–63) years |
|
|
6 (30.0)
(11.9–54.3) |
|
|
|
Kainulainen, L.
(1999) |
Finland |
Cohort |
Turku University Hospital, Turku, Finland |
18 (M = 8,
F = 10) |
Median age = 36 (7–69)
Mean age at diagnosis = 31.5
Mean diagnostic delay = 6.3 years |
14 (77.8) (52.4–93.6) |
5 (29.5) (20.6–39.7) |
10 (55.6) (30.8–78.5) |
|
|
|
Cunningham-Rundles, C.
(1999) |
US |
Case series |
Mount Sinai Medical
Center at Memorial Hospital in New York City, from 1973 to 1986 and at Mount Sinai Medical Center from 1986 to 1999, over a 25-year period |
248 (M = 102, F = 146) |
Median age of onset = F:28 and M:23
Median age of diagnosis = F:29 and M:33 |
190 (76.6)
(70.8–81.7) |
|
|
|
Candida:
3 (1.2)
(0.3–3.5)
Hep C:
14 ( 5.6)
(3.1–9.3)
URTI:
243 (98.0)
(95.4–99.3)
CNS infection:
4 (1.6)
(0.4–4.1) |
|
Nordoy, I.
(1998) |
Norway |
Case-control |
Section of Clinical Immunology and Infectious Diseases, Rikshospitalet |
31 (M = 13,
F = 18) |
- |
|
|
7 (9.7)
(2.0–25.8) |
|
Hep C:
2 (6.5)
(0.8–21.4) |
|
Morris, A.
(1998) |
UK |
|
Medical Research Council Immunodeficiency Clinic, Royal Free Hospital, London |
77 (M = 41,
F = 36) |
Mean age = 46
Mean age at diagnosis = 26.6 |
|
|
|
|
Hep C:
3 (4.3)
(0.9–12.2) |
|
Aukrust, P.
(1996) |
Norway |
Case-control |
- |
24 (M = 9,
F = 15) |
Median age = 38 (21–74) |
|
|
9 (37.5)
(18.8–59.4) |
|
Hep C:
2 (8.3)
(1.0–27.0) |
|
Rump, J. A.
(1995) |
Germany |
Case-control |
- |
15(M = 6,
F = 9) |
Mean age = 44.4±13.8 |
|
|
|
|
SI:
4 (30.8)
(9.1–61.4) |
|
Herbst, E. W.
(1994) |
Germany |
Case-control |
Institute of Pathology
and *Department of Internal Medicine, University of Freiburg |
17 (M = 7,
F = 10) in case (CVID) |
- |
|
|
17 (100)
(80.5–100.0) |
|
Candida:
2 (11.8)
(1.5–36.4)
SI:
2 (11.8)
(1.5–36.4)
Conjunctivitis:
3 (17.6)
(3.8–43.4)
Tonsillitis: 17 (100)
(80.5–100.0) |
|
Singh, Y.
(1994) |
India |
Retrospective cohort |
Clinical Immunology Services of The All
India Institute of Medical Sciences |
14(M = 10,
F = 4) |
Mean age = 12.1 (range: 2–40) |
12 (85.7) (57.2–98.2) |
|
|
|
URTI: 6 (42.9)
(17.7–71.1) |
|
Aukrust, P.
(1994) |
Norway |
Case-control |
Medical Department A, University of Oslo, National Hospital, Rikshospitalet |
25 (M = 9,
F = 16) in case (CVID) |
Age of onset = 2 |
|
|
8 (30)
(11.9–54.3) |
|
Hep C: 1; |
|
Pandolfi, F.
(1993) |
USA |
Case-control |
Department of Allergy and Clinical Immunology, La Sapienza University of Rome |
40 (M = 19,
F = 21)
9 |
Mean age of onset = 28.5 |
8 (88.9)
(51.8–99.7) |
|
6 (66.7)
(29.9–92.5) |
|
Hep C:
1 (2.5)
(0.1–13.2)
URTI:
9 (100)
(66.4–100.0) |
|
Sweinberg, S..
(1991) |
USA |
Retrospective |
Immunology clinic at Children’s Hospital of Philadelphia
between 1975 and 1988 |
12 (M = 6,
F = 6) |
Mean age = 23.5±7.9
Mean age at onset = 8.5±9.8 years
Mean age at diagnosis = 12.5±9.3
Mean diagnostic delay = 4.1±4.2 years |
10 (83.3) (51.6–97.9) |
|
|
|
|
|
Lebranchu, Y.
(1991) |
France |
Case-control |
- |
9 (M = 3.
F = 6) |
Mean age = 16–55 (range = 16–55) |
7 (77.8)
(40.0–97.2) |
|
8 (88.9) (51.8–99.7) |
1 (11.1) (0.3–48.2) |
|
|
Hansel, T.
(1987) |
UK |
Retrospective cohort |
The regional immunology laboratory in Birmingham |
161 (M = 96,
F = 65) |
- |
145 (90.1)
(84.4–94.2) |
|
|
|
URTI: 36 (22.4)
(16.6–29.4)
Sepsis: 27 (16.8)
(11.4–23.5)
Septic arthritis: 3 (1.9)
(0.4–5.3)
CNS infection: 8 (5.0)
(2.2–9.6) |
|
Conley, M..
(1986) |
US |
Case series |
All patients followed at Children’s Hospital of Philadelphia between 1980 and 1985. All patients followed at Children’s Hospital of Philadelphia between 1980 and 1985 |
8 (M = 3,
F = 5) |
Mean age = 14.83
Mean age at onset = 1.78
Mean Age at diagnosis = 5.5 |
5 (62.5)
(24.5–91.5) |
8 (100)
(63.1–100.0) |
7 (87.5)
(47.3–99.7) |
5 (62.5) (24.5–91.5) |
SI: 1 (12.5)
(2.2–47.1)
CNS infection: 1 (12.5)
(0.3–52.7) |
|
Gajl-Peczalska, K.
(1973) |
US |
Case-control |
- |
9 (M = 6,
F = 3) |
Mean age = 29.6 |
8 (88.9)
(51.8–99.7) |
5 (55.6) (21.2–86.3) |
5 (55.6) (21.2–86.3) |
|
SI:1 (11.1)
(2.0–43.5)
Conjunctivitis: 1 (11.1)
(0.3– 48.2) |
PID: primary immunodeficiency; URTI: upper respiratory tract infection; CNS: central nervous system; SI: skin infection; IQR: interquartile range; CI: confidence interval.
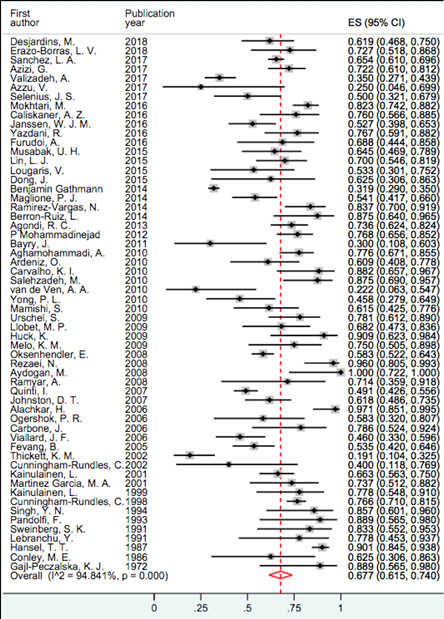
Figure 2: Forest plot and pooled prevalence of pneumonia.
Table 2: Pooled prevalence of various infections among the common variable immunodeficiency patients.
|
Pneumonia |
58 |
3937, 2294 |
19.1–100 |
67.7 (61.5–74.0) |
94.8 |
|
URTI |
20 |
1477, 978 |
12.0–100 |
59.0 (45.5–72.4) |
98.1 |
|
Sinusitis |
44 |
1339, 2187 |
6.3–100 |
57.6 (50.1–65.2) |
93.7 |
|
Otitis media |
32 |
1471, 628 |
10.0–100 |
46.5 (38.3–54.8) |
91.0 |
|
Bacterial infection |
19 |
950, 274 |
5.3–100 |
41.7 (18.8–64.6) |
99.4 |
|
Tonsillitis |
4 |
507, 49 |
5.0–100 |
38.9 (20.3–98.1) |
99.5 |
|
Gastrointestinal infection |
26 |
1635, 409 |
0–87.5 |
36.3 (26.5–46.2) |
97.2 |
|
Viral infection |
26 |
1837, 472 |
1.6–100 |
25.4 (13.0–37.7) |
98.9 |
|
Parasitic infection |
22 |
1344, 163 |
1.9–87.0 |
18.8 (13.5–24.0) |
91.9 |
|
Pharyngitis |
4 |
598, 101 |
7.2–32.0 |
18.8 (9.6–28.1) |
81.2 |
|
Skin infection |
17 |
938, 178 |
2.5–36.4 |
17.1 (11.9 - 22.4) |
71.8 |
|
Abscess |
11 |
1084, 91 |
1.6–100 |
16.9 (10.4–23.3) |
94.4 |
|
Conjunctivitis |
13 |
975, 154 |
9.1–36.0 |
16.9 (12.1–21.7) |
64.7 |
|
CNS infection |
25 |
3141, 192 |
1.3–100 |
11.2 (7.3–15.1) |
96.1 |
|
Sepsis |
14 |
1632, 128 |
1.2–22.2 |
7.3 (4.4–10.3) |
83.0 |
|
Candidiasis |
12 |
1367, 125 |
0.8–42.9 |
7.0 (5.8 –13.9) |
90.3 |
|
Hepatitis C |
14 |
1870, 108 |
1.2–34.9 |
5.7 (3.4–8.0) |
72.8 |
|
Septic arthritis |
9 |
908, 44 |
0.8–24.0 |
5.0 (2.3–7.7) |
75.7 |
|
Fungal infection |
11 |
1458, 51 |
1.1–46.9 |
3.4 (1.4–5.5) |
75.1 |
URTI: upper respiratory tract infection; CNS: central nervous system; CI: confidence intarval; I2: I-square.
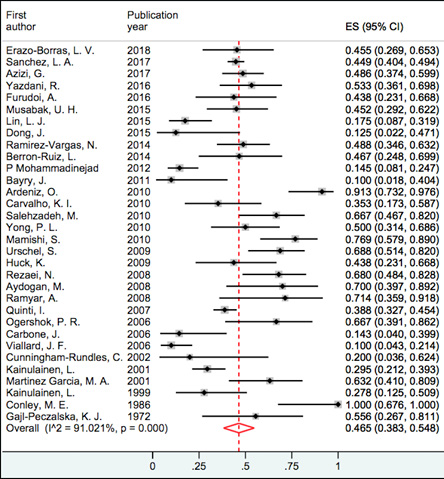
Figure 3: Forest plot and pooled prevalence of otitis media.
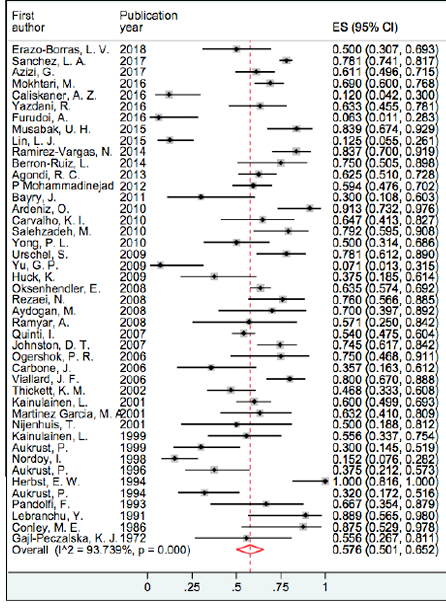
Figure 4: Forest plot and pooled prevalence of sinusitis.
Table 3: Demographic and corresponding immunologic data of CVID patients with and without infection.
|
Sex ratio (M/F), n = 291 |
155/136 |
123/108 |
32/28 |
0.990 |
|
Consanguinity (Yes/No), n = 30 |
18/12 |
16/11 |
2/1 |
1.000 |
|
Age at onset, years median (IQR), n = 49 |
20.0 (20.0) |
14.0 (21.0) |
24.0 (18.2) |
0.296 |
|
Age at diagnosis, years median (IQR), n = 96 |
12.0 (27.0) |
10.0 (13.9) |
28.0 (24.0) |
0.003** |
|
Diagnostic delay, years median (IQR), n = 30 |
4.0 (8.8) |
2.1 (5.3) |
4.0 (8.7) |
0.343 |
|
IgG mg/dL, median (IQR), n = 193 |
276.0 (285.5) |
272.5 (250.2) |
280.0 (326.0) |
0.406 |
|
IgA mg/dL, median (IQR), n = 149 |
9.0 (24.5) |
6.0 (19.4) |
10.0 (32.2) |
0.129 |
|
IgM mg/dL, median (IQR), n = 149 |
10.0 (26.0) |
17.0 (35.0) |
10.0 (23.7) |
0.051* |
|
CD3+ lymphocytes, cell/mL, n = 40 |
947.5 (832.7) |
478.0 (748.7) |
979.0 (678.1) |
0.013** |
|
CD4+ T cells, cell/mL, n = 38 |
550.0 (274.5) |
429.0 (NA) |
550.0 (271.0) |
0.626 |
|
CD8+ T cells, cell/mL, n = 38 |
572.5 (482.7) |
375.0 (NA) |
580.0 (428.0) |
0.570 |
|
CD19+ lymphocytes, cell/mL, n = 65 |
232.0 (237.1) |
283.0 (294.0) |
146.0 (174.6) |
0.027** |
CVID: common variable immunodeficiency; M: male; F: female; IQR: interquartile range; Ig: immunoglobulin.
Note: For age, age at onset, age at diagnosis, delay in diagnosis, the median is shown [with 25th and 75th percentiles]. Mann-Whitney U test for a numerical variable and the chi-square test or Fisher’s exact test for the nominal variable was used.
*p-value is statistically significant < 0.050.
Our findings showed that respiratory tract infection was the most frequent infectious complications in patients with CVID. Pneumonia prevalence was between 19.1% and 100%, and based on random effect model, the pooled prevalence was 67.7% (95% confidence intervals: 61.5–74.0; I2 = 94.8) and it was the most prevalent infection [Figure 2], followed by upper respiratory tract infections (URTIs) with a prevalence of 59.0%. Sinusitis, otitis media, and tonsillitis showed higher prevalence among URTIs with 57.6%, 46.5%, and 38.9%, respectively. Figures 3 and 4 represent the forest plot and pooled prevalence of sinusitis and otitis media. Forest plot of prevalent infections did not show any trend of prevalence over time
[Figures 2, 3, and 4]. Gastrointestinal infection with a prevalence of 36.3% was the second most infectious complications in CVID patients. In contrast, osteomyelitis was the least reported infection among patients with CVID. Additionally, bacterial infections were more reported in CVID patients compared with viral, parasitic, or fungal infections (41.7% vs. 25.4%, 18.8%, or 3.4%, respectively). Detailed information regarding the prevalence of infections are depicted in Table 2.
Based on meta-regression analyses, there were several significant immunological characteristics that explain the following types of infections prevalence. By increasing the diagnosis age by one year, there was a decrease in the prevalence of the said diseases, pneumonia by 1.2%, sinusitis by 2.5%, gastrointestinal infections by 1.6%, and infectious arthritis by 0.9% (p = 0.009, p = 0.006, p = 0.016, and p = 0.018, respectively). Moreover, per 100 mg/dL increase in IgM serum level, the prevalence of hepatitis C and gastrointestinal infections showed a decrease of 6.6% (p = 0.006) and 1.2% (p = 0.090), respectively. Also, per 100 mg/dL increase in IgG serum level, there was a decrease in prevalence of infectious arthritis by 4.4% (p = 0.037), and per 100 cell/mL increase in CD3+ T cells, the prevalence of viral infections showed a decrease of 2.7% (p = 0.016).
In order to obtain more insight into the infectious characteristics of CVID patients, we compared demographic and corresponding immunologic data of CVID patients with and without infections in 24 completely defined studies. These studies comprised a total of 404 patients with CVID, of which 264 patients had a history of at least one known infection. CVID patients with infections showed significantly lower percentage of CD3+ T cells compared to CVID patients without infections (478.0 (748.7) vs. 979.0 (678.1), p = 0.013). Also, the median (IQR) age at diagnosis for CVID patients with infection was 10.0 (13.9) years and was significantly lower than that of CVID patients without infection (p = 0.003). Moreover, the median (IQR) age at onset of symptoms, and IgA and IgM levels in CVID patients having infections were lower than that of patients without infection even though it was not statistically significant. CVID patients with a history of infection had lower percentages of CD4+ and CD8+ T cells compared to CVID patients without infections, although this was not statistically significant. In contrast, higher percentages of CD19+ lymphocytes (283.0 (294.0) vs. 146.0 (174.6), p = 0.027) were found in CVID patients with a history of infections compared to patients without this history. The detailed compared parameters are shown in Table 3.
Discussion
Infections are the main characteristic findings and leading cause of morbidity and mortality in CVID patients. Although, over the past years early diagnosis and therapeutic strategies of CVID have been improved, yet there are accumulating results from epidemiological studies proving a high burden of infectious complications in these patients leading to a high incidence of deaths. Several studies have reported various prevalence rates of different infections; however, almost all of them are quite univocal to the fact that upper and/or lower respiratory infections are the most prevalent infectious complication among these patients. The highest and the lowest prevalence of upper respiratory infections were reported by Martínez García et al,16 and Pandolfi et al,17 at 100% and Çalişkaner et al,18 at 12.0%, respectively. However, the results of meta-analysis showed that the pooled prevalence of URTIs was 59.0%.
Pneumonia is estimated to be the leading cause of lower respiratory infection, morbidity, and mortality globally in CVID patients.84 There is a high prevalence of pneumonia among patients with CVID reported by large cohort studies conducted by Hansel et al,19 Mokhtari et al,20 and Cunningham-Rundles et al,6 with frequencies of 90.1%, 82.3%, and 76.6%, respectively. In this study, pneumonia was assessed and reported in 58 studies, and based on the results of the random effect method, the pooled prevalence of pneumonia in total CVID patients was 67.7%. Therefore, we can conclude that pneumonia is one of the main complications of these patients and, in cases of mismanagement, could cause significant and everlasting further morbidities such as bronchiectasis, which is commonly reported in CVID patients.
We found that the cumulative infections attributable to bacterial etiologies were more frequent in CVID compared to viral or fungal infections (41.7% vs. 25.4% and 3.4%, respectively). Similarly, other studies reported a higher incidence of bacterial infections among CVID patients.3,5,13 The higher prevalence of bacterial pathogens causing infections in these patients can be interpreted as a result of impairment in antibody production by plasma cells. In contrast, cellular immunity is the effective defense mechanism against viral and fungal infections (it is already shown in the inverse correlation between the T lymphocyte count and the viral infections), hence infections caused by viral and fungal pathogens have lower prevalence as cellular immunity is less affected in patients with CVID.
CVID patients with infection had a significantly lower age at diagnosis compared to CVID patients without infection. It could be because the presentation of infection is one of the main characteristic features of the disease that could lead to earlier diagnosis for CVID. Unlike patients presenting with other manifestations of the disease, such as autoimmunity, allergy, and cancers, which are diagnosed in later stages of life.
There was a lower percentage of CD3+ in CVID patients with infection compared to the group of CVID patients without infection. T cells, as well as B cells defect, are associated with more severe disease and higher infection rates in these patients.
Higher percentages of CD19+ lymphocytes in patients with a history of infections might indicate that the primary defect is likely related to impairments of terminal stages of B cell differentiation.85,86 Higher and/or normal numbers of CD19+ does not necessarily indicate a better immune response. The impaired antibody production despite normal B cell counts in a study conducted by Ahn and Cunningham-Rundles.85 Also suggests a defect in the differentiation of B cells into plasma and memory cells in many CVID patients. Several studies have pointed out the decreased level of class switch memory B cells (CD19+/CD27+/IgD-/IgM-), IgM memory B cells (CD19+/CD27+), and plasma cells in patients with CVID disease.87–89 Furthermore, Unger et al,90 reported that a subgroup of CVID patients manifests with the expansion of a special subset of B cells known as CD21low B cells, and it has been demonstrated that its expansion is linked with immune dysregulation in CVID patients.
A broad spectrum of T-cell abnormalities, including total numbers, percentages, surface markers, and function of various T-cell subpopulations have been reported in CVID patients. It has been shown a reduction of the total, naïve and memory CD4+ T cells, recent thymic emigrants, and an increase in activated CD4+ T cells. Some studies have demonstrated that CVID patients with a profound decrease in CD4+ T-cell counts are more susceptible to develop autoimmunity and lymphoproliferative diseases, indicating that there is a strong correlation between the frequency of naïve T cells and clinical manifestations.91,92 Similar to CD4+ T cells, a decline in the frequency of CD8+ T-cell subsets has been demonstrated. Naïve and effector memory CD8+T-cell numbers are reduced whereas higher percentages of activated CD8+ T cells have been reported.61,93,94
Disclosure
The authors declare no conflict of interest. This work was supported by the vice chancellor for research, Alborz University of Medical Sciences (1396-02-12-1500).
Acknowledgements
The authors would like to thank the Clinical Research Development Center of Imam Ali-Karaj Hospital.
references
- 1. Tam JS, Routes JM. Common variable immunodeficiency. Am J Rhinol Allergy 2013 Jul-Aug;27(4):260-265.
- 2. Saikia B, Gupta S. Common variable immunodeficiency. Indian J Pediatr 2016 Apr;83(4):338-344.
- 3. Cunningham-Rundles C. How I treat common variable immune deficiency. Blood 2010 Jul;116(1):7-15.
- 4.aAzizi G, Hafezi N, Mohammadi H, Yazdani R, Alinia T, Tavakol M, et al. Abnormality of regulatory T cells in common variable immunodeficiency. Cell Immunol. 2017;315:11-17.
- Abolhassani H, Kiaee F, Tavakol M, Chavoshzadeh Z, Mahdaviani SA, Momen T, et al. Fourth update on the Iranian National Registry of Primary Immunodeficiencies: Integration of Molecular Diagnosis. J Clin Immunol 2018;38(7):816-832.
- 6. Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999 Jul;92(1):3-4-48.
- 7. Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008 Jul;112(2):277-286.
- 8. Yazdani R, Abolhassani H, Asgardoon MH, Shaghaghi M, Modaresi M, Azizi G, et al. Infectious and noninfectious pulmonary complications in patients with primary immunodeficiency disorders. J Investig Allergol Clin Immunol 2017;27(4):213-224.
- 9. Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al; Italian Primary Immunodeficiency Network. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 2007 May;27(3):308-316.
- 10. Herbst EW, Armbruster M, Rump JA, Buscher HP, Peter HH. Intestinal B cell defects in common variable immunodeficiency. Clin Exp Immunol 1994 Feb;95(2):215-221.
- 11. Rezaei N, Aghamohammadi A, Siadat SD, Moin M, Pourpak Z, Nejati M, et al. Serum bactericidal antibody responses to meningococcal polysaccharide vaccination as a basis for clinical classification of common variable immunodeficiency. Clin Vaccine Immunol 2008 Apr;15(4):607-611.
- 12. Aydogan M, Eifan AO, Gocmen I, Ozdemir C, Bahceciler NN, Barlan IB. Clinical and immunologic features of pediatric patients with common variable immunodeficiency and respiratory complications. J Investig Allergol Clin Immunol 2008;18(4):260-265.
- 13. Lin LJ, Wang YC, Liu XM. Clinical and immunological features of common variable immunodeficiency in China. Chin Med J (Engl) 2015 Feb;128(3):310-315.
- 14. Salehzadeh M, Aghamohammadi A, Rezaei N. Evaluation of immunoglobulin levels and infection rate in patients with common variable immunodeficiency after immunoglobulin replacement therapy. J Microbiol Immunol Infect 2010 Feb;43(1):11-17.
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009 Jul;6(7):e1000097.
- 16. Martínez García MA, de Rojas MD, Nauffal Manzur MD, Muñoz Pamplona MP, Compte Torrero L, Macián V, et al. Respiratory disorders in common variable immunodeficiency. Respir Med 2001 Mar;95(3):191-195.
- 17. Pandolfi F, Paganelli R, Cafaro A, Oliva A, Giovannetti A, Scala E, et al. Abnormalities of lymphocyte subpopulations in CVI do not correlate with increased production of IL-6. Immunodeficiency 1993;4(1-4):19-23.
- 18. Çalişkaner AZ, Reisli İ, Arslan Ş, Uçar R, Ataseven H, Selçuk NY. Common variable immunodeficiency in adults requires reserved protocols for long-term follow-up. Turk J Med Sci 2016 Feb;46(2):430-436.
- 19. Hansel TT, Haeney MR, Thompson RA. Primary hypogammaglobulinaemia and arthritis. Br Med J (Clin Res Ed) 1987 Jul;295(6591):174-175 .
- 20. Mokhtari M, Shakeri A, Mirminachi B, Abolhassani H, Yazdani R, Grimbacher B, et al. Important factors influencing severity of common variable immunodeficiency. Arch Iran Med 2016 Aug;19(8):544-550.
- 21. Desjardins M, Béland M, Dembele M, Lejtenyi D, Drolet JP, Lemire M, et al. Modulation of the interleukin-21 pathway with interleukin-4 distinguishes common variable immunodeficiency patients with more non-infectious clinical complications. J Clin Immunol 2018 Jan;38(1):45-55.
- 22. Erazo-Borrás LV, Álvarez-Álvarez JA, Perez-Romero CA, Orrego-Arango JC, Franco-Restrepo JL, Trujillo-Vargas CM. Skewed invariant natural killer T (iNKT) cells, impaired iNKT:B cell help and decreased SAP expression in blood lymphocytes from patients with common variable immunodeficiency. Scand J Immunol 2017 Sep;86(3):171-178.
- 23. Azzu V, Elias JE, Duckworth A, Davies S, Brais R, Kumararatne DS, et al. Liver transplantation in adults with liver disease due to common variable immunodeficiency leads to early recurrent disease and poor outcome. Liver Transpl 2018 Feb;24(2):171-181.
- 24. Sanchez LA, Maggadottir SM, Pantell MS, Lugar P, Rundles CC, Sullivan KE; USIDNET Consortium. Two sides of the same coin: pediatric-onset and adult-onset common variable immune deficiency. J Clin Immunol 2017 Aug;37(6):592-602.
- 25. Valizadeh A, Yazdani R, Azizi G, Abolhassani H, Aghamohammadi A. A comparison of clinical and immunologic phenotypes in familial and sporadic forms of common variable immunodeficiency. Scand J Immunol 2017 Oct;86(4):239-247.
- 26. Friedmann D, Keller B, Harder I, Schupp J, Tanriver Y, Unger S, et al. Preferential reduction of circulating innate lymphoid cells type 2 in patients with common variable immunodeficiency with secondary complications is part of a broader immune dysregulation. J Clin Immunol 2017 Nov;37(8):759-769.
- 27. Selenius JS, Martelius T, Pikkarainen S, Siitonen S, Mattila E, Pietikäinen R, et al. Unexpectedly high prevalence of common variable immunodeficiency in Finland. Front Immunol 2017 Sep;8(SEP):1190.
- 28. Furudoï A, Gros A, Stanislas S, Hamidou M, Furudoï E, Oksenhendler É, et al. Spleen histologic appearance in common variable immunodeficiency. Am J Surg Pathol 2016 Jul;40(7):958-967.
- 29. Janssen WJ, Mohamed Hoesein F, Van de Ven AA, Maarschalk J, van Royen F, de Jong PA, et al. IgG trough levels and progression of pulmonary disease in pediatric and adult common variable immunodeficiency disorder patients. J Allergy Clin Immunol 2017 Jul;140(1):303-306.e4.
- 30. Kutukculer N, Azarsiz E, Aksu G, Karaca NE. CD4+CD25+Foxp3+ T regulatory cells, Th1 (CCR5, IL-2, IFN-γ) and Th2 (CCR4, IL-4, Il-13) type chemokine receptors and intracellular cytokines in children with common variable immunodeficiency. Int J Immunopathol Pharmacol 2016 Jun;29(2):241-251.
- 31. Yazdani R, Seify R, Ganjalikhani-Hakemi M, Abolhassani H, Eskandari N, Golsaz-Shirazi F, et al. Comparison of various classifications for patients with common variable immunodeficiency (CVID) using measurement of B-cell subsets. Allergol Immunopathol (Madr). 2016.
- 32. Lougaris V, Baronio M, Masneri S, Lorenzini T, Cattivelli K, Tampella G, et al. Correlation of bone marrow abnormalities, peripheral lymphocyte subsets and clinical features in uncomplicated common variable immunodeficiency (CVID) patients. Clin Immunol 2016 Feb;163:10-13 .
- 33. Arshi S, Nabavi M, Bemanian MH, Shakeri R, Taghvaei B, Ghalebaghi B, et al. Phenotyping and follow up of forty-seven Iranian patients with common variable immunodeficiency. Allergol Immunopathol (Madr) 2016 May-Jun;44(3):226-231.
- 34. Dong J, Liang H, Wen D, Wang J. Adult common variable immunodeficiency. Am J Med Sci 2016 Mar;351(3):239-243.
- 35. Gathmann B, Mahlaoui N, Gérard L, Oksenhendler E, Warnatz K, Schulze I, et al; CEREDIH; Dutch WID; European Society for Immunodeficiencies Registry Working Party. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014 Jul;134(1):116-126 .
- 36. Berrón-Ruiz L, López-Herrera G, Vargas-Hernández A, Mogica-Martínez D, García-Latorre E, Blancas-Galicia L, et al. Lymphocytes and B-cell abnormalities in patients with common variable immunodeficiency (CVID). Allergol Immunopathol (Madr) 2014 Jan-Feb;42(1):35-43.
- 37. Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Ann Allergy Asthma Immunol 2014 Oct;113(4):452-459.
- 38. Ramírez-Vargas N, Arablin-Oropeza SE, Mojica-Martínez D, Yamazaki-Nakashimada MA, de la Luz García-Cruz M, Terán-Juárez LM, et al. Clinical and immunological features of common variable immunodeficiency in Mexican patients. Allergol Immunopathol (Madr) 2014 May-Jun;42(3):235-240.
- 39. Agondi RC, Barros MT, Kokron CM, Cohon A, Oliveira AK, Kalil J, et al. Can patients with common variable immunodeficiency have allergic rhinitis? Am J Rhinol Allergy 2013 Mar-Apr;27(2):79-83.
- 40. Mohammadinejad P, Aghamohammadi A, Abolhassani H, Sadaghiani MS, Abdollahzade S, Sadeghi B, et al. Pediatric patients with common variable immunodeficiency: long-term follow-up. J Investig Allergol Clin Immunol 2012;22(3):208-214.
- 41. Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Sibéril S, et al. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun 2011 Feb;36(1):9-15.
- 42. Al-Herz W, Nanda A. Skin manifestations in primary immunodeficient children. Pediatr Dermatol 2011 Sep-Oct;28(5):494-501.
- 43. Malamut G, Verkarre V, Suarez F, Viallard JF, Lascaux AS, Cosnes J, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol 2010 Oct;105(10):2262-2275.
- 44. Ardeniz O, Başoğlu OK, Günşar F, Unsel M, Bayraktaroğlu S, Mete N, et al. Clinical and immunological analysis of 23 adult patients with common variable immunodeficiency. J Investig Allergol Clin Immunol 2010;20(3):222-236.
- 45. Carvalho KI, Melo KM, Bruno FR, Snyder-Cappione JE, Nixon DF, Costa-Carvalho BT, et al. Skewed distribution of circulating activated natural killer T (NKT) cells in patients with common variable immunodeficiency disorders (CVID). PLoS One 2010 Sep;5(9):e12652.
- 46. van de Ven AA, van de Corput L, van Tilburg CM, Tesselaar K, van Gent R, Sanders EA, et al. Lymphocyte characteristics in children with common variable immunodeficiency. Clin Immunol 2010 Apr;135(1):63-71.
- 47. Yong PL, Orange JS, Sullivan KE. Pediatric common variable immunodeficiency: immunologic and phenotypic associations with switched memory B cells. Pediatr Allergy Immunol 2010 Aug;21(5):852-858.
- 48. Mamishi S, Eghbali AN, Rezaei N, Abolhassani H, Parvaneh N, Aghamohammadi A. A single center 14 years study of infectious complications leading to hospitalization of patients with primary antibody deficiencies. Braz J Infect Dis 2010 Jul-Aug;14(4):351-355.
- 49. Huck K, Feyen O, Ghosh S, Beltz K, Bellert S, Niehues T. Memory B-cells in healthy and antibody-deficient children. Clin Immunol 2009 Apr;131(1):50-59.
- 50. Urschel S, Kayikci L, Wintergerst U, Notheis G, Jansson A, Belohradsky BH. Common variable immunodeficiency disorders in children: delayed diagnosis despite typical clinical presentation. J Pediatr 2009 Jun;154(6):888-894.
- 51. Yu GP, Chiang D, Song SJ, Hoyte EG, Huang J, Vanishsarn C, et al. Regulatory T cell dysfunction in subjects with common variable immunodeficiency complicated by autoimmune disease. Clin Immunol 2009 May;131(2):240-253.
- 52. Melo KM, Carvalho KI, Bruno FR, Ndhlovu LC, Ballan WM, Nixon DF, et al. A decreased frequency of regulatory T cells in patients with common variable immunodeficiency. PLoS One 2009 Jul;4(7):e6269.
- 53. Malphettes M, Gérard L, Carmagnat M, Mouillot G, Vince N, Boutboul D, et al; DEFI Study Group. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis 2009 Nov;49(9):1329-1338.
- 54. Ramyar A, Aghamohammadi A, Moazzami K, Rezaei N, Yeganeh M, Cheraghi T, et al. Presence of Idiopathic Thrombocytopenic Purpura and autoimmune hemolytic anemia in the patients with common variable immunodeficiency. Iran J Allergy Asthma Immunol 2008 Sep;7(3):169-175.
- 55. Sève P, Bourdillon L, Sarrot-Reynauld F, Ruivard M, Jaussaud R, Bouhour D, et al; DEF-I Study Group. Autoimmune hemolytic anemia and common variable immunodeficiency: a case-control study of 18 patients. Medicine (Baltimore) 2008 May;87(3):177-184.
- 56. Ward C, Lucas M, Piris J, Collier J, Chapel H. Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol 2008 Sep;153(3):331-337.
- 57. Johnston DT, Schroeder HW Jr. B-cell numbers in the blood of patients with non-HLA*B8 or non-HLA*B44 common variable immunodeficiency. Ann Allergy Asthma Immunol 2007 Feb;98(2):163-167.
- 58. Khodadad A, Aghamohammadi A, Parvaneh N, Rezaei N, Mahjoob F, Bashashati M, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 2007 Nov;52(11):2977-2983.
- 59. Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol 2006 Sep;120(3):310-318.
- 60. Ogershok PR, Hogan MB, Welch JE, Corder WT, Wilson NW. Spectrum of illness in pediatric common variable immunodeficiency. Ann Allergy Asthma Immunol 2006 Nov;97(5):653-656.
- 61. Carbone J, Sarmiento E, Micheloud D, Rodríguez-Molina J, Fernández-Cruz E. Elevated levels of activated CD4 T cells in common variable immunodeficiency: association with clinical findings. Allergol Immunopathol (Madr) 2006 Jul-Aug;34(4):131-135.
- 62. Viallard JF, Blanco P, André M, Etienne G, Liferman F, Neau D, et al. CD8+HLA-DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clin Immunol 2006 Apr;119(1):51-58.
- 63. Fevang B, Mollnes TE, Holm AM, Ueland T, Heggelund L, Damås JK, et al. Common variable immunodeficiency and the complement system; low mannose-binding lectin levels are associated with bronchiectasis. Clin Exp Immunol 2005 Dec;142(3):576-584.
- 64. Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM 2002 Oct;95(10):655-662 .
- 65. Cunningham-Rundles C, Cooper DL, Duffy TP, Strauchen J. Lymphomas of mucosal-associated lymphoid tissue in common variable immunodeficiency. Am J Hematol 2002 Mar;69(3):171-178.
- 66. Guazzi V, Aiuti F, Mezzaroma I, Mazzetta F, Andolfi G, Mortellaro A, et al. Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles. Clin Exp Immunol 2002 Aug;129(2):346-353.
- 67. Quinti I, Pierdominici M, Marziali M, Giovannetti A, Donnanno S, Chapel H, et al; European Study Group for the Surveillance of Immunoglobulin Safety. European surveillance of immunoglobulin safety–results of initial survey of 1243 patients with primary immunodeficiencies in 16 countries. Clin Immunol 2002 Sep;104(3):231-236.
- 68. Kainulainen L, Nikoskelainen J, Ruuskanen O. Diagnostic findings in 95 Finnish patients with common variable immunodeficiency. J Clin Immunol 2001 Mar;21(2):145-149.
- 69. Nijenhuis T, Klasen I, Weemaes CM, Preijers F, de Vries E, van der Meer JW. Common variable immunodeficiency (CVID) in a family: an autosomal dominant mode of inheritance. Neth J Med 2001 Sep;59(3):134-139.
- 70. Bjøro K, Skaug K, Haaland T, Frøland SS. Long-term outcome of chronic hepatitis C virus infection in primary hypogammaglobulinaemia. QJM 1999 Aug;92(8):433-441.
- 71. Aukrust P, Aandahl EM, Skålhegg BS, Nordøy I, Hansson V, Taskén K, et al. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol 1999 Jan;162(2):1178-1185.
- 72. Kainulainen L, Varpula M, Liippo K, Svedström E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 1999 Nov;104(5):1031-1036.
- 73. Nordøy I, Müller F, Aukrust P, Frøland SS. Adhesion molecules in common variable immunodeficiency (CVID)–a decrease in L-selectin-positive T lymphocytes. Clin Exp Immunol 1998 Nov;114(2):258-263.
- 74. Morris A, Webster AD, Brown D, Harrison TJ, Dusheiko G. GB virus C infection in patients with primary antibody deficiency. J Infect Dis 1998 Jun;177(6):1719-1722.
- 75. Aukrust P, Lien E, Kristoffersen AK, Müller F, Haug CJ, Espevik T, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency–possible immunologic and clinical consequences. Blood 1996 Jan;87(2):674-681 .
- 76. Rump JA, Jakschiess D, Walker U, Schlesier M, von Wussow P, Peter HH. Common variable immunodeficiency (CVID) and MxA-protein expression in blood leucocytes. Clin Exp Immunol 1995 Jul;101(1):89-93.
- 77. Singh YN, Khare SD, Malaviya AN. Common variable immunodeficiency (CVID) in northern India. Asian Pac J Allergy Immunol 1994 Dec;12(2):169-172.
- 78. Aukrust P, Müller F, Frøland SS. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency (CVI) are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin Immunol Immunopathol 1994 Mar;70(3):217-224.
- 79. Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J Allergy Clin Immunol 1991 Jul;88(1):96-104.
- 80. Lebranchu Y, Thibault G, Degenne D, Bardos P. Abnormalities in CD4+ T lymphocyte subsets in patients with common variable immunodeficiency. Clin Immunol Immunopathol 1991 Oct;61(1):83-92.
- 81. Conley ME, Park CL, Douglas SD. Childhood common variable immunodeficiency with autoimmune disease. J Pediatr 1986 Jun;108(6):915-922.
- 82. Gajl-Peczalska KJ, Park BH, Biggar WD, Good RA. B and T lymphocytes in primary immunodeficiency disease in man. J Clin Invest 1973 Apr;52(4):919-928.
- 83. Muşabak UH, Demirel F, Yeşillik S, Baysan A, Selçuk A, Kartal Ö, et al. Adults with common variable immunodeficiency: a single-center experience. Turk J Med Sci 2017 Feb;47(1):1-12.
- 84. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018 Nov;18(11):1191-1210.
- 85. Ahn S, Cunningham-Rundles C. Role of B cells in common variable immune deficiency. Expert Rev Clin Immunol 2009 Sep;5(5):557-564.
- 86. Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom 2008 Sep;74(5):261-271.
- 87. Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol 2005 Feb;115(2):412-417.
- 88. Warnatz K, Denz A, Dräger R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 2002 Mar;99(5):1544-1551.
- 89. Piqueras B, Lavenu-Bombled C, Galicier L, Bergeron-van der Cruyssen F, Mouthon L, Chevret S, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol 2003 Sep;23(5):385-400.
- 90. Unger S, Seidl M, van Schouwenburg P, Rakhmanov M, Bulashevska A, Frede N, et al. The TH1 phenotype of follicular helper T cells indicates an IFN-γ-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol 2018 Feb;141(2):730-740.
- 91. Giovannetti A, Pierdominici M, Mazzetta F, Marziali M, Renzi C, Mileo AM, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol 2007 Mar;178(6):3932-3943.
- 92. Vlkova M, Ticha O, Nechvatalova J, Kalina T, Litzman J, Mauri C, et al. Regulatory B cells in CVID patients fail to suppress multifunctional IFN-γ+ TNF-α+ CD4+ T cells differentiation. Clin Immunol 2015 Oct;160(2):292-300.
- 93. Bateman EA, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol 2012 Nov;170(2):202-211.
- 94. Viallard JF, Ruiz C, Guillet M, Pellegrin JL, Moreau JF. Perturbations of the CD8(+) T-cell repertoire in CVID patients with complications. Results Immunol 2013 May;3:122-128.